NEUROSCIENCE:
A Bite to Remember
Catharine H. Rankin
Simple learning in animals can be induced by four types of experimental protocol--habituation, sensitization, classical conditioning, and instrumental conditioning (see the figure). In classical conditioning, an animal learns about stimuli that predict important events such as food or danger. An example is Pavlov's dog who learned that the sound of a bell predicts the delivery of food. In instrumental conditioning, an animal learns that a particular behavior has a specific consequence--for example, a rat learns to push a lever in order to get food.
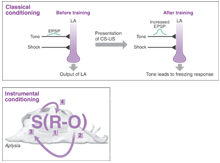
Figure: Classical and instrumental conditioning. (Top) One form of classical conditioning involves conditioning animals to fear a stimulus (7). In the example shown, animals are conditioned to expect an electric shock (unconditioned stimulus, US) when they hear an auditory tone (conditioned stimulus, CS). The neurons involved in learning this connection are situated in the lateral amygdala (LA), which instigates a conditioned freezing response when the tone is heard. The cellular mechanism underlying this learned behavior is a change in the activity of NMDA receptors at specific synapses (long-term potentiation) that then increase AMPA-type glutamate receptor currents in lateral amygdala neurons. (Bottom) Instrumental conditioning in the sea slug Aplysia (4). During instrumental conditioning, a stimulus (S) results in a response (R) that leads to an expected outcome (O), usually a reward. (1) The sea slug exhibits spontaneous biting behavior. (2) Stimulation of the esophageal nerve responsible for ingestion leads to dopamine release onto the B51 sensory neuron. (3) If dopamine is applied to B51 contingent on esophageal nerve activity and biting behavior, there is a change in the resting potential and excitability of B51. (4) This change increases the probability that a biting response will occur.
A critical issue concerns whether each form of learning is truly unique or whether they represent artificial categories imposed by researchers. Dissecting this problem is complicated by the fact that most experimental protocols induce two or more forms of learning (1, 2). For example, classical conditioning contains an instrumental component because the response (salivation at the sound of the bell) is rewarded by the important event (food). Similarly, during instrumental conditioning, the setting and cues associated with training lead to a form of classical conditioning called context conditioning, which tells the animal what to expect in that environment (pressing the lever leads to food delivery) (3). But behavioral experiments alone cannot tell us conclusively about the relationships among the different forms of learning; we also need to understand the cellular mechanisms underlying each of them. On page 1706 of this issue, Brembs et al. (4) take a step in this direction with their analysis of the behavior of the sea slug Aplysia californica during instrumental conditioning. By investigating the behavior of the animal and correlating it with the activity of single neurons, these authors were able to unravel a dopamine reward pathway resembling that in mammals.
What we know about the cellular mechanisms of learning is primarily based on work using sensitization (5) and classical conditioning protocols (5-7). In contrast, the cellular mechanisms underlying habituation and instrumental conditioning are poorly understood. Instrumental conditioning presents a particularly tricky problem to understand at a mechanistic level. Modern theorists (3) see the contingencies constituting instrumental conditioning as S(R-O), which means that in the presence of a specific stimulus (S), a response (R) leads to an expected outcome or reward (O). To determine the changes in neural activity (plasticity) that accompany instrumental learning, researchers need to understand the neural pathways underlying each of the elements of the contingency (S, R, and O).
Byrne and his colleagues (8, 9) have developed a way to study instrumental conditioning in Aplysia both in vitro and in vivo. Their protocol examines the biting phase of the feeding response in Aplysia, which can occur spontaneously. The esophageal nerve normally carries sensory feedback during food ingestion. By stimulating the esophageal nerve directly, spontaneous biting behavior can be reinforced even in the absence of food (4). An examination of the nervous system of trained animals shows that training alters the biophysical properties of the B51 neuron. The B51 sensory neuron is important for determining the output of the buccal motor system that regulates biting (8, 9). This neuron seems to be the point of convergence between the biting response and reinforcement. Using an in vitro system, Brembs et al. (4) applied the neurotransmitter dopamine to cultured B51 neurons each time they fired in a pattern that mimicked ingestion. As a result of this reinforcement, the biophysical properties of the B51 neuron changed, rendering it more excitable and more likely to fire. This led to an increase in the frequency of ingestion-like firing patterns of B51.
In mammals, dopamine is known to be crucial for instrumental conditioning [reviewed in (10)]. More specifically, dopamine is the key neurotransmitter mediating the reward-seeking behaviors trained by this type of conditioning. Dopamine may mediate approach behaviors activated by stimuli that are associated with biologically relevant events. The Brembs et al. work adds a new dimension to this research by presenting a simple system in which the effects of dopamine on single neurons, and on the behavior produced by those neurons, can be studied.
In the rat, release of dopamine in the nucleus accumbens can be selectively gated by the sensory properties of food, which in turn are gated by hunger and novelty (11). This suggests that food reinforcement delivered to a hungry rat leads to the release of dopamine, which provides the incentive motivating repetition of the behavior that produced the food (10, 11). In Aplysia, it is B51 that influences the buccal motor system to carry out ingestion (8). Repeated application of dopamine to B51 after it fires with an ingestion-like pattern alters the excitability of this neuron, leading to a higher probability of ingestion-like patterns. It is noteworthy that Brembs et al. artificially delivered dopamine to B51 every time it fired with an ingestion-like pattern. It will be important to further investigate the neural pathway that connects food intake, the esophageal nerve, and B51 to determine which stimuli are necessary and sufficient for stimulating natural release of dopamine contingent on biting, and to analyze the types of neural plasticity that dopamine induces.
Mechanisms of classical conditioning are highly conserved across a broad range of species (5, 7). Now, thanks to the work of Brembs and colleagues, it is clear that the mechanisms underlying instrumental conditioning may also be highly conserved. The next step will be to see whether any common cellular mechanisms underlie classical and instrumental conditioning.
References
- M. Domjan, The Principles of Learning and Behavior (Brooks/Cole, Pacific Grove, CA, 1998).
- S. R. Coleman, I. Gormezano, Behaviorism 7, 1 (1979).
- R. A. Rescorla, Q. J. Exp. Psychol. 43B, 1 (1991).
- B. Brembs et al., Science 296, 1706 (2002).
- E. R. Kandel, Science 294, 1030 (2001).
- Murphy et al., J. Neurosci. 19, 10595 (1999).
- H. T. Blair et al., Learn. Mem. 8, 229 (2001).
- R. Nargeot, D. A. Baxter, J. H. Byrne, J. Neurosci. 19, 2247 (1999).
- ------, J. Neurosci. 19, 2261 (1999).
- S. Ikemoto, J. Panksepp, Brain Res. Rev. 31, 6 (1999).
- S. Ahn, A. G. Phillips, J. Neurosci. 19, RC29 1 (1999).
The author is in the Department of Psychology and Brain Research Centre, University of British Columbia, Vancouver, BC V6T 1Z4, Canada. E-mail: crankin@cortex.psych.ubc.ca