|
Full citation: Brembs, B and Hempel de Ibarra, N (2006): Different parameters support generalization and discrimination learning in Drosophila at the flight simulator. Learn. Mem. 2006;13 629-637 |
Different parameters support generalization and discrimination learning in Drosophila at the flight simulator
1 Institute of Biology and Neurobiology, Freie Universität Berlin, 14195 Berlin, Germany; , 2 School of Life Sciences, University of Sussex, Falmer, Brighton, BN1 9QG, United Kingdom |
|
| LEARNING & MEMORY 13:629-637 ISSN 1072-0502/06 $5.00 |
||
| Google Scholar | |
| Articles by Brembs, B. | |
| Articles by Hempel de Ibarra, N. | |
| PubMed | |
| Articles by Brembs, B. | |
| Articles by Hempel de Ibarra, N. | |
| ABSTRACT |
|---|
| |
|---|
We have used a genetically tractable model system, the fruit fly Drosophila melanogaster to study the interdependence between sensory processing and associative processing on learning performance. We investigated the influence of variations in the physical and predictive properties of color stimuli in several different operant-conditioning procedures on the subsequent learning performance. These procedures included context and stimulus generalization as well as color, compound, and conditional discrimination (colors and patterns). A surprisingly complex dependence of the learning performance on the colors’ physical and predictive properties emerged, which was clarified by taking into account the fly-subjective perception of the color stimuli. Based on estimates of the stimuli’s color and brightness values, we propose that the different tasks are supported by different parameters of the color stimuli; generalization occurs only if the chromaticity is sufficiently similar, whereas discrimination learning relies on brightness differences.
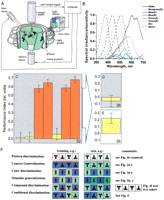
All animals extract relevant cues from the continuum of the incoming sensory stream to learn about their environment and how to behave in it. But how are the salient, predictive cues extracted from this stream and what factors determine the composition of a memory template? Obviously, some things are learned faster and remembered better than others. The relative timing of stimuli is of course paramount (for discussion, see Brembs and Wiener 2006![]() ). Another factor could be the physical make-up of a predictive stimulus. For example, it is usually assumed that a conspicuous, localized stimulus will be easier to learn than a diffuse, extended background stimulus. But is this seemingly straightforward insight true for all sorts of memory templates? In this study, we use colors and patterns in the visual learning paradigms for Drosophila melanogaster at the flight simulator to approach this problem.
). Another factor could be the physical make-up of a predictive stimulus. For example, it is usually assumed that a conspicuous, localized stimulus will be easier to learn than a diffuse, extended background stimulus. But is this seemingly straightforward insight true for all sorts of memory templates? In this study, we use colors and patterns in the visual learning paradigms for Drosophila melanogaster at the flight simulator to approach this problem.
There is only limited evidence that Drosophila uses and learns color as visual cue (Quinn et al. 1974![]() ; Spatz et al. 1974
; Spatz et al. 1974![]() ; Menne and Spatz 1977
; Menne and Spatz 1977![]() ; Bicker and Reichert 1978
; Bicker and Reichert 1978![]() ; Desalomon and Spatz 1983
; Desalomon and Spatz 1983![]() ). On the other hand, colors have been used as stimuli in a number of studies involving visual-discrimination learning in the flight simulator (Wolf and Heisenberg 1997
). On the other hand, colors have been used as stimuli in a number of studies involving visual-discrimination learning in the flight simulator (Wolf and Heisenberg 1997![]() ; Wolf et al. 1998
; Wolf et al. 1998![]() ; Brembs and Heisenberg 2001
; Brembs and Heisenberg 2001![]() ; Tang and Guo 2001
; Tang and Guo 2001![]() ), where visual patterns are presented on the inner wall of a vertical cylinder (arena) surrounding the tethered fly. The yaw torque signal generated by the fly can rotate the arena such that the animal can stabilize the panorama and choose flight direction with respect to the patterns. The coloration of the arena as pattern background can be changed by passing the light through appropriate filters before it reaches the arena (Fig. 1A). While there is a large body of work concerning the processing and learning of patterns in the arena (e.g., Wolf and Heisenberg 1991
), where visual patterns are presented on the inner wall of a vertical cylinder (arena) surrounding the tethered fly. The yaw torque signal generated by the fly can rotate the arena such that the animal can stabilize the panorama and choose flight direction with respect to the patterns. The coloration of the arena as pattern background can be changed by passing the light through appropriate filters before it reaches the arena (Fig. 1A). While there is a large body of work concerning the processing and learning of patterns in the arena (e.g., Wolf and Heisenberg 1991![]() , 1995
, 1995![]() , 1997
, 1997![]() , 1998
, 1998![]() ; Wolf et al. 1992
; Wolf et al. 1992![]() , 1998
, 1998![]() ; Dill et al. 1993
; Dill et al. 1993![]() , 1995
, 1995![]() ; Dill and Heisenberg 1995
; Dill and Heisenberg 1995![]() ; Heisenberg 1995
; Heisenberg 1995![]() ; Guo et al. 1996
; Guo et al. 1996![]() ; Guo and Götz 1997
; Guo and Götz 1997![]() ; Xia et al. 1997a
; Xia et al. 1997a![]() , b
, b![]() , 1999
, 1999![]() ; Gong et al. 1998
; Gong et al. 1998![]() ; Liu et al. 1998
; Liu et al. 1998![]() , 1999
, 1999![]() ; Wang et al. 1998
; Wang et al. 1998![]() , 2003
, 2003![]() ; Ernst and Heisenberg 1999
; Ernst and Heisenberg 1999![]() ; Brembs and Heisenberg 2000
; Brembs and Heisenberg 2000![]() , 2001
, 2001![]() ; Heisenberg et al. 2001
; Heisenberg et al. 2001![]() ; Tang and Guo 2001
; Tang and Guo 2001![]() ; van Swinderen and Greenspan 2003
; van Swinderen and Greenspan 2003![]() ; Greenspan and van Swinderen 2004
; Greenspan and van Swinderen 2004![]() ; Tang et al. 2004
; Tang et al. 2004![]() ; Guo and Guo 2005
; Guo and Guo 2005![]() ), very little is known about the processing of the colors. After the initial discovery that flies learn colors in the flight simulator (Wolf and Heisenberg 1997
), very little is known about the processing of the colors. After the initial discovery that flies learn colors in the flight simulator (Wolf and Heisenberg 1997![]() ), Liu et al. (1999)
), Liu et al. (1999)![]() used background coloration as a context cue during pattern-discrimination learning and found that context generalization depends critically on the spectra of the colors used. Specifically, if the spectra of the two background colors used as context did not overlap fully, flies did not generalize pattern memory between them, whereas colors with full spectral overlap supported context generalization.
used background coloration as a context cue during pattern-discrimination learning and found that context generalization depends critically on the spectra of the colors used. Specifically, if the spectra of the two background colors used as context did not overlap fully, flies did not generalize pattern memory between them, whereas colors with full spectral overlap supported context generalization.
Brembs and Heisenberg (2001)![]() studied the effects of combining colors and patterns in compound stimuli that flies were able to learn. In a chance discovery, we now found a pair of color stimuli with very peculiar effects ("Rosco" blue and green; Fig. 1B). When these colors were presented as background together with black patterns, such a compound of cues was not learned by the flies (Fig. 1C,D). Usually, with two cues as predictive stimuli, such situations can be solved very well by the flies (Brembs and Heisenberg 2001
studied the effects of combining colors and patterns in compound stimuli that flies were able to learn. In a chance discovery, we now found a pair of color stimuli with very peculiar effects ("Rosco" blue and green; Fig. 1B). When these colors were presented as background together with black patterns, such a compound of cues was not learned by the flies (Fig. 1C,D). Usually, with two cues as predictive stimuli, such situations can be solved very well by the flies (Brembs and Heisenberg 2001![]() ). It is important to emphasize that the patterns alone are sufficient predictors, so the flies could disregard the colors and still be able to solve the task. Even more curiously, if after compound training the pattern memory was tested without the "Rosco" colors, it appeared as if it had only been suppressed by the presence of the colors (Fig. 1E). Interestingly, the spectra of these colors overlap only partially, whereas those used previously did either overlap fully or did not overlap at all. This provided us with an excellent opportunity to systematically characterize the relationship between the physical properties of the colors and the associative processes underlying color learning. Inspired by the conclusions from our companion paper, we decided to study the colors in two different generalization tasks and in three discrimination tasks (Fig. 1F) by setting them up as context, conditioned stimuli (CS), and as occasion setters (OS) (Brembs and Wiener 2006
). It is important to emphasize that the patterns alone are sufficient predictors, so the flies could disregard the colors and still be able to solve the task. Even more curiously, if after compound training the pattern memory was tested without the "Rosco" colors, it appeared as if it had only been suppressed by the presence of the colors (Fig. 1E). Interestingly, the spectra of these colors overlap only partially, whereas those used previously did either overlap fully or did not overlap at all. This provided us with an excellent opportunity to systematically characterize the relationship between the physical properties of the colors and the associative processes underlying color learning. Inspired by the conclusions from our companion paper, we decided to study the colors in two different generalization tasks and in three discrimination tasks (Fig. 1F) by setting them up as context, conditioned stimuli (CS), and as occasion setters (OS) (Brembs and Wiener 2006![]() ).
).
| Results |
|---|
| |
|---|
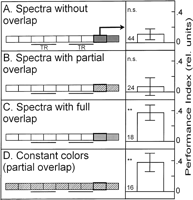
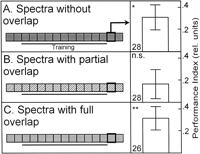
It has been established previously that flies that have been trained to discriminate between two visual patterns with background illumination in one color and tested for context generalization by presenting the same patterns in a different color background do not show the conditioned pattern discrimination when the spectrum of the test color does not overlap with that of the training color (Fig. 2A; from Liu et al. 1999![]() ). However, if the spectrum of one of the colors is fully contained within the spectrum of the second (i.e., they exhibit full overlap of their spectra), the pattern memory is generalized across color contexts and in both directions of the reciprocal arrangements (Fig. 2C; Liu et al. 1999
). However, if the spectrum of one of the colors is fully contained within the spectrum of the second (i.e., they exhibit full overlap of their spectra), the pattern memory is generalized across color contexts and in both directions of the reciprocal arrangements (Fig. 2C; Liu et al. 1999![]() ). Interestingly, when the flies are trained in one color and tested in another, the spectrum of which partially overlaps with that of the training color, flies again do not show the conditioned discrimination (Fig. 2B). Thus, colors with nonoverlapping or only partially overlapping spectra do not support context generalization. This effect cannot be attributed to the colors themselves, as patterns are used by the flies for the conditioned avoidance when the colors are kept constant throughout the experiment (Fig. 2D). Importantly, these experiments show that the flies distinguish the two colors with partially overlapping spectra; otherwise the flies would show the conditioned pattern preference in the new color. Apparently, lack of discrimination cannot be the explanation for the failure to learn the pattern/color compound cue (Fig. 1D).
). Interestingly, when the flies are trained in one color and tested in another, the spectrum of which partially overlaps with that of the training color, flies again do not show the conditioned discrimination (Fig. 2B). Thus, colors with nonoverlapping or only partially overlapping spectra do not support context generalization. This effect cannot be attributed to the colors themselves, as patterns are used by the flies for the conditioned avoidance when the colors are kept constant throughout the experiment (Fig. 2D). Importantly, these experiments show that the flies distinguish the two colors with partially overlapping spectra; otherwise the flies would show the conditioned pattern preference in the new color. Apparently, lack of discrimination cannot be the explanation for the failure to learn the pattern/color compound cue (Fig. 1D).
|
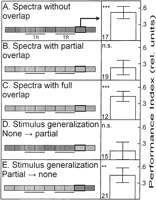
However, to get an idea of the degree to which the colors in the three color pairs differ, we compared how flies discriminate color pairs with no, partial, or complete spectral overlap when they are set up as operant CSs (see Fig. 1C). While colors with full or no overlap in their spectra can be discriminated very well (Fig. 3A,C), flies do not show conditioned discrimination after training with colors that show only partial overlap in their spectra (Fig. 3B). From these results alone, one usually would conclude that flies cannot discriminate colors with partial overlap of their spectra. But the context generalization experiments suggest the opposite. One hypothesis explaining these contradictory results obtained with the partially overlapping colors may be that colors with partially overlapping spectra can be distinguished by the flies but not sufficiently as to support discrimination learning. To test this hypothesis, we asked whether flies can generalize a conditioned discrimination between partially overlapping colors to the pair with nonoverlapping spectra and vice-versa (Fig. 3; see Fig. 1). The prediction was that if flies only distinguish the partially overlapping colors, but do not learn them, we should not find any generalization. If, on the other hand, the flies can both distinguish and learn the partially overlapping colors, we may find generalization from the partially overlapping colors to the nonoverlapping colors. Indeed, we found stimulus generalization, but only in one direction; when colors with no overlap are trained (i.e., to discriminate between Kodak green and blue) and then the flies are tested with the partially overlapping color pair (i.e., whether they discriminate Rosco green and blue), no significant performance index is obtained (Fig. 3D). However, if the inverse situation is invoked, the flies trained to distinguish partially overlapping colors show a generalized conditioned discrimination. The flies preferred the unpunished color of the nonoverlapping color pair during the test phase (i.e., Kodak blue if Rosco green was punished and vice versa; Fig. 3E). In conclusion, the flies discriminate partially overlapping colors and generalize their conditioned color preference to the nonoverlapping colors. However, retrieval of the conditioned preference is not directly guided by the perceptual difference between the partially overlapping colors. Following the same line of argument, we can conclude that flies acquired a conditioned color preference even during color and pattern-color compound discrimination training with partially overlapping colors, but failed to retrieve this preference with these colors.
|
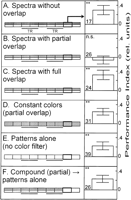
Combining operant pattern and color-discrimination learning to compound-discrimination learning (see also Fig. 1C,D,E), we studied the interaction of the two stimuli (Fig. 4). The results mimic those obtained in the color-discrimination experiments, i.e., colors with full or no overlap in their spectra support compound discrimination (Fig. 4A,C), whereas flies do not show conditioned discrimination after compound training in which the patterns were presented together with background colors that show partial overlap in their spectra (Fig. 4B). It needs to be pointed out that training the flies with the patterns alone, i.e., on a background illuminated by white light without color filters, is sufficient to enable the flies to choose the right flight direction (Fig. 4E). In other words, the successive presence of the colors with partially overlapping spectra disrupts the pattern discrimination normally taking place. Importantly, it is not the spectral restriction per se that disrupts pattern discrimination, as pattern-discrimination learning is evident if the background is colored in one of the two overlapping colors, but kept constant (Figs. 2D, 4D). An important control procedure is to remove the overlapping color filters after compound training, presenting the patterns in white light. Flies expressed a significant pattern preference during this test, revealing the dominant effect of the partially overlapping colors in the retrieval of conditioned pattern preferences (Fig. 4F).
|
Finally, to provide further evidence that our results are task (discrimination vs. generalization) and not paradigm specific, we tested the latest and most complex paradigm at the flight simulator, conditional discrimination, a form of occasion setting (see Fig. 1). In this paradigm, the colors serve as a higher-order predictor, indicating the nature of the pattern/heat contingency (Brembs and Wiener 2006![]() ). Specifically, background coloration of one color indicates that the upright T is being punished and the other color indicates that the inverted T is being punished. Again paralleling our previous results, both colors with completely overlapping spectra and colors with nonoverlapping spectra support conditional discrimination (Fig. 5A,C), whereas colors with partially overlapping spectra do not (Fig. 5B).
). Specifically, background coloration of one color indicates that the upright T is being punished and the other color indicates that the inverted T is being punished. Again paralleling our previous results, both colors with completely overlapping spectra and colors with nonoverlapping spectra support conditional discrimination (Fig. 5A,C), whereas colors with partially overlapping spectra do not (Fig. 5B).
|
Puzzled by this unexpected complexity in our results, we decided to characterize the colors from the fly’s perceptual point of view (Table 1). The receptors R1–R6 mediate achromatic coding of visual information, whereas R7 and R8 encode chromaticity. The non- and fully overlapping colors showed a large difference in quantum catches for the R1–R6 receptors, thus being clearly different in brightness for the flies, whereas the partially overlapping colors were not (Table 1).
|
Estimation of chromaticity is more difficult. It is generally agreed that R7 and R8 receptors feed into color-coding mechanisms; however, the exact contribution of the two subtypes of R7 and R8 receptors belonging to two ommatidial types is still unknown. Experiments by Troje (1993)![]() and Fukushi (1985
and Fukushi (1985![]() , 1989
, 1989![]() ) with Lucilia indicate that both subtypes may be involved. Since in our experiments the UV range was not used, the R8 receptors were the most strongly excited ones (Fig. 1). We calculated chromaticities using the input of either all four receptor types (Table 1, R7–R8) or alternatively discarding any signal of the very weakly excited R7 receptor with Rh3 opsin (Table 1, Rh4–Rh6). Also, we looked at the predictions of a hypothesized ommatidial opponency mechanism for each of the R7/R8 combinations following an assumption of the processing model by Troje (1993).
) with Lucilia indicate that both subtypes may be involved. Since in our experiments the UV range was not used, the R8 receptors were the most strongly excited ones (Fig. 1). We calculated chromaticities using the input of either all four receptor types (Table 1, R7–R8) or alternatively discarding any signal of the very weakly excited R7 receptor with Rh3 opsin (Table 1, Rh4–Rh6). Also, we looked at the predictions of a hypothesized ommatidial opponency mechanism for each of the R7/R8 combinations following an assumption of the processing model by Troje (1993).![]() Predictions arising from these calculations are not uniform (Table 1). The best correlation to the behavioral results is achieved by calculating color differences from the three strongly excited receptors (Rh4–Rh6) and the ommatidia carrying the Rh4/Rh6 combination. Smaller distances are predicted and calculated for spectra of similar shape that were generalized by the flies, such as the Blue Rosco and Blue Kodak, Green Rosco and Green Kodak, or the other fully overlapping spectra. Larger distances are calculated for spectra of dissimilar shape, e.g., non- and partially overlapping colors, which were not generalized by the flies.
Predictions arising from these calculations are not uniform (Table 1). The best correlation to the behavioral results is achieved by calculating color differences from the three strongly excited receptors (Rh4–Rh6) and the ommatidia carrying the Rh4/Rh6 combination. Smaller distances are predicted and calculated for spectra of similar shape that were generalized by the flies, such as the Blue Rosco and Blue Kodak, Green Rosco and Green Kodak, or the other fully overlapping spectra. Larger distances are calculated for spectra of dissimilar shape, e.g., non- and partially overlapping colors, which were not generalized by the flies.
Classifying our stimuli according to the two perceptual qualities, we establish two subjective axes (Fig. 6); color pairs line up on a brightness difference gradient, in which the partially overlapping colors differ the least in brightness, the nonoverlapping pair differs more, and the fully overlapping pairs differ most in brightness (Fig. 6A). Along the chromaticity axis, the pairs line up with the fully overlapping color pairs showing the smallest chromaticity difference, the partially overlapping pair showing clearly more difference, and the nonoverlapping pair having the largest chromaticity difference (Fig. 6B).
|
| Discussion |
|---|
| |
|---|
In this study, we characterized the functional relationship between the physical properties of three sets of color stimuli and the associative processes underlying color learning in two generalization and three discrimination learning tasks. We found that the color pair with partially overlapping spectra had a number of surprising properties. These colors do not prevent the acquisition of pattern memory, but rather the retrieval of it. Moreover, the partially overlapping colors can be distinguished and learned, but the learned preference cannot be retrieved with these colors present. Judging from all three color pairs’ spectra alone, one would classify the nonoverlapping one as most different, the fully overlapping colors as most similar, and the partially overlapping colors somewhere in-between. One would expect to find a fairly simple system, where generalization and discrimination are steady functions of similarity with inverted signs. Instead, we found a complex set of results that were highly dependent on the spectral properties of the colors used, but where the physical properties alone could not explain all of the variability.
The generalization experiments are in line with the simple expectations; only the color pairs classified as most similar (the ones with full spectral overlap) support the generalization of pattern memory across two contexts characterized by these colors (Fig. 2C). Context generalization was not detected if the background colors were characterized by partially overlapping spectra, indicating that these colors can be distinguished (Fig. 2B). Moreover, color memory acquired during training with these partially overlapping colors alone can generalize to colors without spectral overlap (Fig. 3E), the spectra of which are fully contained within the spectra of the partially overlapping colors. However, the simple predictions are not met in the discrimination experiments. The same colors (with partial overlap), although being distinguishable, do not support conditioned discrimination (Figs. 3B, 5B) and even prevent the retrieval of pattern memory when they are combined with the patterns during compound conditioning (Fig. 4B,F). In contrast, both the most similar colors (with full overlap) and the most different colors (without overlap) support all of our discrimination learning tasks.
This demonstrates the interaction between sensory processing (distinguishing between the colors) and associative processing (forming a memory template); in the context generalization experiment, the partially overlapping colors are incorporated into the memory template and prevent generalization (much like the nonoverlapping colors), but in the discrimination tasks (color and compound discrimination learning, conditional discrimination) they are not sufficiently incorporated to support retrieval of the memory. Yet, colors that are not incorporated into the memory template in a context generalization experiment (i.e., the colors with fully overlapping spectra) support discrimination learning just fine.
Hoping that the key to understanding such complicated results may lie in the subjective perceptual quality of color stimuli, we computed the flies’ perception of the colors (Fig. 6). The percept of a color is influenced by the physical function of light intensity and wavelength distribution. These basic properties can be encoded as brightness and color cues, which are commonly processed in parallel neural systems and mediate different perceptual functions (Livingstone and Hubel 1988![]() ; Gegenfurtner and Kiper 2003
; Gegenfurtner and Kiper 2003![]() ; Osorio and Vorobyev 2005
; Osorio and Vorobyev 2005![]() ). Thus, colors carry both chromaticity and brightness cues that may result in a unique percept, but can also mediate different parts of the behavioral output (Lehrer 1987
). Thus, colors carry both chromaticity and brightness cues that may result in a unique percept, but can also mediate different parts of the behavioral output (Lehrer 1987![]() ; Hempel de Ibarra et al. 2002
; Hempel de Ibarra et al. 2002![]() ; Kelber et al. 2003
; Kelber et al. 2003![]() ; Kelber 2005
; Kelber 2005![]() ). From such computations (Fig. 6), we derive the most parsimonious hypothesis that Drosophila extracts different spectral parameters from the color stimuli to solve the generalization and the discrimination tasks, respectively. For generalization across two colors, they must be sufficiently similar in their chromaticity. In the fully overlapping color pairs, which are the most similar in terms of chromaticity, generalization occurs despite even relatively large brightness differences (Fig. 2C). Larger differences in chromaticity are sufficient to prevent generalization, despite only small brightness differences and some spectral overlap (Fig. 2B). So far, these results conform to the simple "similarity" expectation. For conditioned discrimination of two colors (Figs. 3, 4, 5), neither the spectral overlap, nor the chromaticity difference, nor the strength of background brightness with a stronger or weaker contrast to the black pattern can account for our complex set of results. Instead, it appears that there has to be a large brightness difference for two colors to support any of our kinds of discrimination learning. Colors with only partial spectral overlap, large color differences, but only small brightness differences can be distinguished by the flies (Figs. 2B, 3E), but spoil the test (Figs. 3B, 5B) and prevent the retrieval of pattern memory (Fig. 4B,F). Apparently, similarity in brightness is sufficient to prevent the three kinds of discrimination learning used in this study, even if other parameters differ widely. Interestingly, the chromaticity difference between the two partially overlapping colors can be learned, but it can only be retrieved with colors that differ also in brightness (Fig. 3E). Thus, the failure in discrimination learning of partially overlapping colors is attributable to a failure in retrieval, rather than acquisition.
). From such computations (Fig. 6), we derive the most parsimonious hypothesis that Drosophila extracts different spectral parameters from the color stimuli to solve the generalization and the discrimination tasks, respectively. For generalization across two colors, they must be sufficiently similar in their chromaticity. In the fully overlapping color pairs, which are the most similar in terms of chromaticity, generalization occurs despite even relatively large brightness differences (Fig. 2C). Larger differences in chromaticity are sufficient to prevent generalization, despite only small brightness differences and some spectral overlap (Fig. 2B). So far, these results conform to the simple "similarity" expectation. For conditioned discrimination of two colors (Figs. 3, 4, 5), neither the spectral overlap, nor the chromaticity difference, nor the strength of background brightness with a stronger or weaker contrast to the black pattern can account for our complex set of results. Instead, it appears that there has to be a large brightness difference for two colors to support any of our kinds of discrimination learning. Colors with only partial spectral overlap, large color differences, but only small brightness differences can be distinguished by the flies (Figs. 2B, 3E), but spoil the test (Figs. 3B, 5B) and prevent the retrieval of pattern memory (Fig. 4B,F). Apparently, similarity in brightness is sufficient to prevent the three kinds of discrimination learning used in this study, even if other parameters differ widely. Interestingly, the chromaticity difference between the two partially overlapping colors can be learned, but it can only be retrieved with colors that differ also in brightness (Fig. 3E). Thus, the failure in discrimination learning of partially overlapping colors is attributable to a failure in retrieval, rather than acquisition.
These results are intriguing with respect to the results described in our companion paper (Brembs and Wiener 2006![]() ). Their data indicate that the predictive relationship to the reinforcer is the decisive factor of whether a context is incorporated into the memory template (context dependence or discrimination) or not (context independence or generalization). Our data suggest that extraction of different parameters of the background stimulus (context) underlies generalization and discrimination, respectively. One might speculate also that different neural substrates support generalization and discrimination, respectively, and that it is the combination of physical and predictive properties of the "context" that determines whether or not any given memory will be context dependent or independent. In this view, it makes little sense to study context dependence (discrimination) or independence (generalization) without determining the source of the phenomenon in terms of the physical (this study) and the predictive (Brembs and Wiener 2006
). Their data indicate that the predictive relationship to the reinforcer is the decisive factor of whether a context is incorporated into the memory template (context dependence or discrimination) or not (context independence or generalization). Our data suggest that extraction of different parameters of the background stimulus (context) underlies generalization and discrimination, respectively. One might speculate also that different neural substrates support generalization and discrimination, respectively, and that it is the combination of physical and predictive properties of the "context" that determines whether or not any given memory will be context dependent or independent. In this view, it makes little sense to study context dependence (discrimination) or independence (generalization) without determining the source of the phenomenon in terms of the physical (this study) and the predictive (Brembs and Wiener 2006![]() ) properties of the stimuli in question.
) properties of the stimuli in question.
| Materials and Methods |
|---|
| |
|---|
Flies
Flies are kept on standard cornmeal/molasses medium (Guo et al. 1996![]() ) at 25°C and 60% humidity with a 14-h light/10-h dark regime. Females aged 24–48 h are briefly immobilized by cold anesthesia and glued (Loctite UV glass glue) with head and thorax to a triangle-shaped copper hook (diameter 0.05 mm) the day before the experiment. The animals are then kept individually overnight in small moist chambers containing a few grains of sucrose.
) at 25°C and 60% humidity with a 14-h light/10-h dark regime. Females aged 24–48 h are briefly immobilized by cold anesthesia and glued (Loctite UV glass glue) with head and thorax to a triangle-shaped copper hook (diameter 0.05 mm) the day before the experiment. The animals are then kept individually overnight in small moist chambers containing a few grains of sucrose.
Spectral stimuli
Three pairs of color filters were used (see Fig. 1B). (1) Filters with nonoverlapping spectra—broad-band blue (No. 47) and broad-band green (No. 99) Kodak Wratten gelatin filter. (2) Filters with partially overlapping spectra—Rosco "just blue" (No. 079) and Rosco "dark green" (No. 124). (3) Filters with fully overlapping spectra—"Daylight" blue-green (Rosco "surfblue" No. 5433) with either the Kodak green or the Kodak blue filter. The transmission spectrum of the Rosco blue-green filter used in this study is equivalent to that of the BG18 filter (Schott, Mainz) used by Liu et al. (1999)![]() (data not shown). Light spectra were measured inside the arena using a calibrated photospectrometer (SD 2000, Ocean Optics). To calculate photoreceptor excitations as integral of the spectrum of a white light source filtered through different color filters and spectral receptor sensitivities (Wyszecki and Stiles 1982
(data not shown). Light spectra were measured inside the arena using a calibrated photospectrometer (SD 2000, Ocean Optics). To calculate photoreceptor excitations as integral of the spectrum of a white light source filtered through different color filters and spectral receptor sensitivities (Wyszecki and Stiles 1982![]() ), we used template-absorbance spectra (Stavenga et al. 1993
), we used template-absorbance spectra (Stavenga et al. 1993![]() ) of Drosophila rhodopsins for its known sensitivity peaks (
) of Drosophila rhodopsins for its known sensitivity peaks (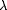 max of 480, 347, 375, 436, and 508 nm, Rh1 and Rh3–Rh6, respectively) (Feiler et al. 1988
max of 480, 347, 375, 436, and 508 nm, Rh1 and Rh3–Rh6, respectively) (Feiler et al. 1988![]() , 1992
, 1992![]() ; Salcedo et al. 2003
; Salcedo et al. 2003![]() ). Chromatic differences of the color stimuli were determined using the Receptor Noise Limited model of color vision (Vorobyev and Osorio 1998
). Chromatic differences of the color stimuli were determined using the Receptor Noise Limited model of color vision (Vorobyev and Osorio 1998![]() ; Hempel de Ibarra et al. 2000
; Hempel de Ibarra et al. 2000![]() ), which has been successfully applied to tri- or tetrachromatic visual systems of human, birds, and bees, respectively (Vorobyev and Osorio 1998
), which has been successfully applied to tri- or tetrachromatic visual systems of human, birds, and bees, respectively (Vorobyev and Osorio 1998![]() ; Hempel de Ibarra et al. 2000
; Hempel de Ibarra et al. 2000![]() ). The input to color-opponent mechanisms was assumed to be either through all four R7 and R8 receptor cells or separately through each of the two R7/R8 units belonging to different ommatidia (Rh3/Rh5, Rh4/Rh6) (Chou et al. 1999
). The input to color-opponent mechanisms was assumed to be either through all four R7 and R8 receptor cells or separately through each of the two R7/R8 units belonging to different ommatidia (Rh3/Rh5, Rh4/Rh6) (Chou et al. 1999![]() ). The UV range was excluded in our light stimuli, thus Rh3 was hardly excited at all, which allows calculation of a trichromatic input from Rh4–Rh6. Since the presented color pairs were well above their discrimination thresholds, a common value was used as noise estimate in R7/R8 receptor cells (Weber fraction of 0.1) (c.f. Vorobyev et al. 1998
). The UV range was excluded in our light stimuli, thus Rh3 was hardly excited at all, which allows calculation of a trichromatic input from Rh4–Rh6. Since the presented color pairs were well above their discrimination thresholds, a common value was used as noise estimate in R7/R8 receptor cells (Weber fraction of 0.1) (c.f. Vorobyev et al. 1998![]() , 2001
, 2001![]() ). We assumed its independence from the spectral channel and included a differential input of receptors based on a distribution ratio of 1:2.4 of the different ommatidia for the two ommatidial types Rh3/Rh5 and Rh4/Rh6 (Stark and Thomas 2004
). We assumed its independence from the spectral channel and included a differential input of receptors based on a distribution ratio of 1:2.4 of the different ommatidia for the two ommatidial types Rh3/Rh5 and Rh4/Rh6 (Stark and Thomas 2004![]() ). Fly-subjective brightness was estimated through the quantum catch of the R1–R6 (Rh1) receptors (Heisenberg and Buchner 1977
). Fly-subjective brightness was estimated through the quantum catch of the R1–R6 (Rh1) receptors (Heisenberg and Buchner 1977![]() ; Hardie 1986
; Hardie 1986![]() ; Anderson and Laughlin 2000
; Anderson and Laughlin 2000![]() ). The perceptual differences of the color stimuli are listed in Table 1.
). The perceptual differences of the color stimuli are listed in Table 1.
Apparatus
The Drosophila flight simulator is a computer-controlled feedback system; the fly uses its yaw torque to control the rotations of a panorama surrounding it. The core device is the torque meter (Götz 1964![]() ; Heisenberg and Wolf 1984
; Heisenberg and Wolf 1984![]() ), which measures a fly’s angular momentum around its vertical body axis. The fly, glued to the hook, is attached to the torque meter via a clamp to accomplish stationary flight in the center of a cylindrical panorama (arena; diameter 58 mm) homogeneously illuminated from behind (Fig. 1A). The light source is a 100W, 12V tungsten-iodine bulb. For background coloration of the arena, the light is passed through one of the different filters described above. Filters can be exchanged by a fast solenoid within 0.1 sec.
), which measures a fly’s angular momentum around its vertical body axis. The fly, glued to the hook, is attached to the torque meter via a clamp to accomplish stationary flight in the center of a cylindrical panorama (arena; diameter 58 mm) homogeneously illuminated from behind (Fig. 1A). The light source is a 100W, 12V tungsten-iodine bulb. For background coloration of the arena, the light is passed through one of the different filters described above. Filters can be exchanged by a fast solenoid within 0.1 sec.
A computer-controlled electric motor rotates the arena such that its angular velocity is proportional to, but directed against the fly’s yaw torque (coupling factor K = –11°/sec·10–10 Nm). This enables the fly to stabilize the panorama and to control its angular orientation. This virtual "flight direction" (i.e., arena position) is recorded continuously via a circular potentiometer (Novotechnik, A4102a306). An analog to digital converter card (PCL812; Advantech Co.) feeds arena position and yaw torque into a computer that stores the traces (sampling frequency 20 Hz) for later analysis.
Punishment is achieved by applying heat from an adjustable infrared laser (825 nm, 150 mW), directed from behind and above onto the fly’s head and thorax. The laser beam is pulsed ( 200 msec pulse width at
200 msec pulse width at  4 Hz) and its intensity reduced to assure the survival of the fly.
4 Hz) and its intensity reduced to assure the survival of the fly.
General experimental design
Each fly is used only once. The time course of the experiment is divided into consecutive periods of either 1- or 2-min duration. Depending on whether heat is applied during such a period, it is termed a training period (heat on) or a test period (heat off). The treatment of the flies during these periods determines the type of experiment, as described below.
Discrimination learning—patterns
For patterns as CS (Wolf and Heisenberg 1991![]() ), four black, T-shaped patterns of alternating orientation (i.e., two upright and two inverted) are evenly spaced on the arena wall (pattern width
), four black, T-shaped patterns of alternating orientation (i.e., two upright and two inverted) are evenly spaced on the arena wall (pattern width 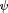 = 40°, height
= 40°, height 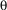 = 40°, width of bars = 14°, as seen from the position of the fly). A computer program divides the 360° of the arena into four virtual 90° quadrants, the centers of which are denoted by the patterns. During training periods, heat punishment is made contiguous with the appearance of one of the pattern orientations in the frontal visual field. Reinforcement of each pattern is always equalized within groups. During test periods, the heat is permanently switched off (see Fig. 1C; pattern learning).
= 40°, width of bars = 14°, as seen from the position of the fly). A computer program divides the 360° of the arena into four virtual 90° quadrants, the centers of which are denoted by the patterns. During training periods, heat punishment is made contiguous with the appearance of one of the pattern orientations in the frontal visual field. Reinforcement of each pattern is always equalized within groups. During test periods, the heat is permanently switched off (see Fig. 1C; pattern learning).
Discrimination learning—colors
For colors as CS (Wolf and Heisenberg 1997![]() ) the centers of the four virtual quadrants are denoted by four identical vertical stripes (width
) the centers of the four virtual quadrants are denoted by four identical vertical stripes (width 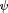 = 14°, height
= 14°, height 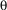 = 40°). The color of the illumination of the whole arena is changed whenever one of the virtual quadrant borders passes a point in front of the fly. During training periods, heat punishment is made contiguous with one of the colors. Reinforcement of each color is always equalized within groups. During test periods, the heat is permanently switched off (see Fig. Fig. 1C; color learning).
= 40°). The color of the illumination of the whole arena is changed whenever one of the virtual quadrant borders passes a point in front of the fly. During training periods, heat punishment is made contiguous with one of the colors. Reinforcement of each color is always equalized within groups. During test periods, the heat is permanently switched off (see Fig. Fig. 1C; color learning).
Discrimination learning—color/pattern compound
If a compound of patterns on a colored background is used as visual cue, the four T-shaped patterns are used and the color is changed as described (Brembs and Heisenberg 2001![]() ). During training periods, heat punishment is made contiguous with both the appearance of one of the pattern orientations in the frontal visual field and with the concomitant change in arena illumination. Reinforcement of each pattern/color is always equalized within groups. During test periods, the heat is permanently switched off (see Fig. 1C; compound discrimination).
). During training periods, heat punishment is made contiguous with both the appearance of one of the pattern orientations in the frontal visual field and with the concomitant change in arena illumination. Reinforcement of each pattern/color is always equalized within groups. During test periods, the heat is permanently switched off (see Fig. 1C; compound discrimination).
Discrimination learning—conditional discrimination (occasion setting)
In this paradigm, arena coloration is used to indicate the nature of the pattern-heat contingency. For instance, flying toward the upright T is punished under green illumination and the inverted T is unpunished, but then blue illumination indicates the reverse pattern-heat contingency. In this experiment, neither of the stimuli alone can unambiguously predict reinforcement. Only the combination of the stimuli is predictive of the heat. In this paradigm, the flies control both colors and patterns operantly. The 360° of the arena are still divided into four virtual 90° quadrants as before. The center of each quadrant is also still denoted by the patterns (alternating upright and inverted Ts). The difference consists of the arrangement of color and heat with the quadrants. While heat was associated with two opposite quadrants (e.g., the ones with the upright T in the center) before, heat is now associated with adjacent quadrants (i.e., one with an upright and one with an inverted T). Thus, instead of being switched on or off at each of the four quadrant borders, the heat is now switched on or off at only two opposite borders. The color of the arena illumination is changed at the remaining two opposite quadrant borders, where the heat is not switched on or off. Thus, heat is applied in two quadrants, which include an upright and an inverted T as well as the quadrant border where the background coloration is changed. Conversely, arena coloration is changed exactly between the two punished patterns and between the two unpunished patterns. In such a way, heat is applied when the flies fly toward, say, a green upright T and a blue inverted T and switch the heat off by flying into one of the other two quadrants with a green inverted T and a blue upright T. One arrangement of quadrants may thus look as follows: The first quadrant features the upright T and whenever the fly enters this quadrant, the whole arena turns to blue illumination. The second quadrant features the inverted T and the arena illumination remains blue. If the fly enters the third quadrant with the upright T, the whole arena turns to green. In the fourth quadrant, the inverted T is in the center, but the arena illumination stays green. The heat regime is such that neither pattern nor color alone could predict punishment. For example, heat is switched on whenever the fly enters quadrants 2 or 3, but no heat is presented when entering quadrants 1 or 4. This heat regime is used for half of the animals, whereas the other half of the animals is not punished in quadrants 2 and 3, but quadrants 1 and 4 are punished (see Fig. 1C; conditional discrimination).
The training phase lasts 11 min and is divided into 1-min periods. After each period, the arena is set to a random position to minimize conditioning to spurious spatial cues. The spatial arrangement of patterns and colors was randomized across periods (i.e., if the patterns in quadrants 1 and 2 were "blue" and the patterns in quadrants 3 and 4 "green" in one period, this association was reversed in a random selection of other periods). This randomization minimized the spatial contingency and emphasized the logical contingency between patterns, heat, and colors. After 11 min of training, the animals are tested for 1 min for their quadrant preference with the heat permanently switched off.
Context generalization
Pattern discrimination training is conducted as described above, albeit with one of the color filters providing constantly colored background illumination of the entire arena. Following the original context generalization experiment by Liu et al. (1999)![]() , only one color change takes place after seven 2-min periods (2 x test, 2 x training, test, 2 x training), introducing a novel background color to the 2-min test period after the last training. For each color pair, the order of the training-test change in color is balanced across animals. A successful context generalization experiment is characterized by a positive learning score, which indicates that the pattern memory was generalized across the different color contexts. Such a successful experiment also shows that the pattern can be processed independently from the color, and the two stimuli are not perceived as a compound (Brembs and Heisenberg 2001
, only one color change takes place after seven 2-min periods (2 x test, 2 x training, test, 2 x training), introducing a novel background color to the 2-min test period after the last training. For each color pair, the order of the training-test change in color is balanced across animals. A successful context generalization experiment is characterized by a positive learning score, which indicates that the pattern memory was generalized across the different color contexts. Such a successful experiment also shows that the pattern can be processed independently from the color, and the two stimuli are not perceived as a compound (Brembs and Heisenberg 2001![]() ). Context generalization is different from context conditioning, where the animals learn to respond to a context. In this study, we never performed context conditioning, but only tested for the ability of a context change to disrupt the transfer of operant pattern memory between contexts. Successful context generalization is characterized by a continued conditioned pattern preference despite the context change (see Fig. 1C; context generalization).
). Context generalization is different from context conditioning, where the animals learn to respond to a context. In this study, we never performed context conditioning, but only tested for the ability of a context change to disrupt the transfer of operant pattern memory between contexts. Successful context generalization is characterized by a continued conditioned pattern preference despite the context change (see Fig. 1C; context generalization).
Stimulus generalization
Color-discrimination training is conducted as described above. At the same point in the experiment as in context generalization, the color filters are exchanged to a different pair of filters. Then, color preference is tested with the heat permanently switched off, testing for color-discrimination learning during the 2-min test period after the last training (see Fig. 1C; stimulus generalization).
Data evaluation and statistics
The color and/or pattern preference of individual flies is calculated as the performance index: PI = (ta-tb)/(ta+tb). During training periods, tb indicates the time the fly is exposed to the heat and ta the time without heat. During test periods, ta and tb refer to the times when the fly chose the formerly (or subsequently) unpunished or punished situation, respectively. Thus, a PI of 1 means the fly spent the entire period in the quadrants not associated with heat, whereas a PI of –1 indicates that the fly spent the entire period in the quadrants associated with heat. Accordingly, a PI of 0 indicates that the fly distributed the time evenly between heated and nonheated quadrants. PI’s from test periods are called "test PIs" or "learning scores." Learning scores were tested for significance using a t-test for single means against zero, following Liu et al. (1999)![]() .
.
| Acknowledgments |
|---|
This work was supported by the DFG (BR 1893/3-2).
| FOOTNOTES |
|---|
3 Corresponding author.
E-mail bjoern@brembs.net
; fax 49-308-385-5455. ![]()
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.319406
| References |
|---|
| |
|---|
Anderson, J.C. and Laughlin, S.B. 2000. Photoreceptor performance and the co-ordination of achromatic and chromatic inputs in the fly visual system. Vision Res. 40: 13–31. [CrossRef][Medline]
Bicker, G. and Reichert, H. 1978. Visual learning in a photoreceptor degeneration mutant of Drosophila melanogaster.. J. Comp. Physiol. 127: 29–38.
Brembs, B. and Heisenberg, M. 2000. The operant and the classical in conditioned orientation in Drosophila melanogaster at the flight simulator. Learn. Mem. 7: 104–115.
Brembs, B. and Heisenberg, M. 2001. Conditioning with compound stimuli in Drosophila melanogaster in the flight simulator. J. Exp. Biol. 204: 2849–2859.
Brembs, B. and Wiener, J. 2006. Context generalization and occasion setting: Mushroom bodies stabilize visual memory in Drosophila.. Learn. Mem. (this issue).
Chou, W.H., Huber, A., Bentrop, J., Schulz, S., Schwab, K., Chadwell, L.V., Paulsen, R., Britt, S.G. 1999. Patterning of the r7 and r8 photoreceptor cells of Drosophila: Evidence for induced and default cell-fate specification. Development 126: 607–616. [Abstract]
Desalomon, C.H. and Spatz, H.C. 1983. Color-vision in Drosophila melanogaster—Wavelength discrimination. J. Comp. Physiol. 150: 31–37.
Dill, M. and Heisenberg, M. 1995. Visual pattern memory without shape recognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 349: 143–152. [Medline]
Dill, M., Wolf, R., Heisenberg, M. 1993. Visual pattern recognition in Drosophila involves retinotopic matching. Nature 365: 751–753. [CrossRef][Medline]
Dill, M., Wolf, R., Heisenberg, M. 1995. Behavioral analysis of Drosophila landmark learning in the flight simulator. Learn. Mem. 2: 152–160. [CrossRef][Medline]
Ernst, R. and Heisenberg, M. 1999. The memory template in Drosophila pattern vision at the flight simulator. Vision Res. 39: 3920–3933. [CrossRef][Medline]
Feiler, R., Harris, W.A., Kirschfeld, K., Wehrhahn, C., Zuker, C.S. 1988. Targeted misexpression of a Drosophila opsin gene leads to altered visual function. Nature 333: 737–741. [CrossRef][Medline]
Feiler, R., Bjornson, R., Kirschfeld, K., Mismer, D., Rubin, G.M., Smith, D.P., Socolich, M., Zuker, C.S. 1992. Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: Visual physiology and photochemistry of transgenic animals. J. Neurosci. 12: 3862–3868. [Abstract]
Fukushi, T. 1985. Visual learning in walking blowflies, lucilia cuprina.. J. Comp. Physiol. [A] 157: 771–778. [CrossRef][Medline]
Fukushi, T. 1989. Learning and discrimination of coloured papers in the walking blowfly, lucilia cuprina.. J. Comp. Physiol. [A] 166: 57–64. [Medline]
Gegenfurtner, K.R. and Kiper, D.C. 2003. Color vision. Annu. Rev. Neurosci. 26: 181–206. [CrossRef][Medline]
Gong, Z., Xia, S., Liu, L., Feng, C., Guo, A. 1998. Operant visual learning and memory in Drosophila mutants dunce, amnesiac and radish. J. Insect Physiol. 44: 1149–1158. [CrossRef][Medline]
Götz, K.G. 1964. Optomotorische Untersuchung des Visuellen Systems einiger Augenmutanten der Fruchtfliege Drosophila.. Kybernetik 2: 77–92. [CrossRef][Medline]
Greenspan, R.J. and van Swinderen, B. 2004. Cognitive consonance: Complex brain functions in the fruit fly and its relatives. Trends Neurosci. 27: 707–711. [CrossRef][Medline]
Guo, A. and Götz, K.G. 1997. Association of visual objects and olfactory cues in Drosophila.. Learn. Mem. 4: 192–204. [Abstract]
Guo, J. and Guo, A. 2005. Crossmodal interactions between olfactory and visual learning in Drosophila.. Science 309: 307–310.
Guo, A., Liu, L., Xia, S.-Z., Feng, C.-H., Wolf, R., Heisenberg, M. 1996. Conditioned visual flight orientation in Drosophila; dependence on age, practice, and diet. Learn. Mem. 3: 49–59. [Abstract]
Hardie, R.C. 1986. The photoreceptor array of the dipteran retina. Trends Neurosci. 9: 419–423.
Heisenberg, M. 1995. Pattern recognition in insects. Curr. Opin. Neurobiol. 5: 475–481. [CrossRef][Medline]
Heisenberg, M. and Buchner, E. 1977. Role of retinula cell-types in visual behavior of Drosophila melanogaster.. J. Comp. Physiol. 117: 127–162.
Heisenberg, M. and Wolf, R. 1984. Vision in Drosophila.. In Genetics of microbehavior . Springer, Berlin, Heidelberg, New York, Tokyo.
Heisenberg, M., Wolf, R., Brembs, B. 2001. Flexibility in a single behavioral variable of Drosophila.. Learn. Mem. 8: 1–10.
de Hempel Ibarra, N., Vorobyev, M., Brandt, R., Giurfa, M. 2000. Detection of bright and dim colours by honeybees. J. Exp. Biol. 203: 3289–3298. [Abstract]
-512.de Hempel Ibarra, N., Giurfa, M., Vorobyev, M. 2002. Discrimination of coloured patterns by honeybees through chromatic and achromatic cues. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 188: 503. [CrossRef][Medline]
Kelber, A. 2005. Alternative use of chromatic and achromatic cues in a hawkmoth. Proc. Biol. Sci. 272: 2143–2147.
Kelber, A., Vorobyev, M., Osorio, D. 2003. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. Camb. Philos. Soc. 78: 81–118. [CrossRef][Medline]
Lehrer, M. 1987. To be or not to be a colour-seeing bee. Israel J. Entomol. 21: 51–76.
Liu, L., Wang, X., Xia, S.Z., Feng, C.H., Guo, A. 1998. Conditioned visual flight orientation in Drosophila melanogaster abolished by benzaldehyde. Pharmacol. Biochem. Behav. 61: 349–355. [CrossRef][Medline]
Liu, L., Wolf, R., Ernst, R., Heisenberg, M. 1999. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature 400: 753–756. [CrossRef][Medline]
Livingstone, M. and Hubel, D. 1988. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science 240: 740–749.
Menne, D. and Spatz, H.C. 1977. Color-vision in Drosophila melanogaster.. J. Comp. Physiol. 114: 301–312.
Osorio, D. and Vorobyev, M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: Adaptations for luminance and colour vision. Proc. Biol. Sci. 272: 1745–1752.
Quinn, W.G., Harris, W.A., Benzer, S. 1974. Conditioned behavior in Drosophila melanogaster.. Proc. Natl. Acad. Sci. 71: 708–712.
Salcedo, E., Zheng, L., Phistry, M., Bagg, E.E., Britt, S.G. 2003. Molecular basis for ultraviolet vision in invertebrates. J. Neurosci. 23: 10873–10878.
Spatz, H.C., Emanns, A., Reichert, H. 1974. Associative learning of Drosophila melanogaster.. Nature 248: 359–361. [CrossRef][Medline]
Stark, W.S. and Thomas, C.F. 2004. Microscopy of multiple visual receptor types in Drosophila.. Mol. Vis. 10: 943–955. [Medline]
Stavenga, D.G., Smits, R.P., Hoenders, B.J. 1993. Simple exponential functions describing the absorbance bands of visual pigment spectra. Vision Res. 33: 1011–1017. [CrossRef][Medline]
Tang, S. and Guo, A. 2001. Choice behavior of Drosophila facing contradictory visual cues. Science 294: 1543–1547.
Tang, S.M., Wolf, R., Xu, S.P., Heisenberg, M. 2004. Visual pattern recognition in Drosophila is invariant for retinal position. Science 305: 1020–1022.
Troje, N. 1993. Spectral categories in the learning-behavior of blowflies. Zeitschrift Fur Naturforschung C-a J. Biosci. 48: 96–104.
van Swinderen, B. and Greenspan, R.J. 2003. Salience modulates 20-30 hz brain activity in Drosophila.. Nat. Neurosci. 6: 579–586. [CrossRef][Medline]
Vorobyev, M. and Osorio, D. 1998. Receptor noise as a determinant of colour thresholds. Proc. Biol. Sci. 265: 351–358.
Vorobyev, M., Osorio, D., Bennett, A.T., Marshall, N.J., Cuthill, I.C. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. [A] 183: 621–633. [CrossRef][Medline]
Vorobyev, M., Brandt, R., Peitsch, D., Laughlin, S.B., Menzel, R. 2001. Colour thresholds and receptor noise: Behaviour and physiology compared. Vision Res. 41: 639–653. [CrossRef][Medline]
Wang, X., Liu, L., Xia, S.Z., Feng, C.H., Guo, A. 1998. Relationship between visual learning/memory ability and brain camp level in Drosophila.. Sci. China C Life Sci. 41: 503–511.
Wang, S., Li, Y., Feng, C., Guo, A. 2003. Dissociation of visual associative and motor learning in Drosophila at the flight simulator. Behav. Processes 64: 57–70. [CrossRef][Medline]
Wolf, R. and Heisenberg, M. 1991. Basic organization of operant behavior as revealed in Drosophila flight orientation. J. Comp. Physiol. [A] 169: 699–705. [Medline]
Wolf, R. and Heisenberg, M. 1995. Learning of Drosophila in the flight simulator: Classically conditioned visual pattern discrimination. In Nervous systems and behaviour (eds. Burrows, M. et al.) . pp. 184. Georg Thieme Verlag, Stuttgart, New York Proceedings of the 4th International Congress of Neuroethology.
Wolf, R. and Heisenberg, M. 1997. Visual space from visual motion: Turn integration in tethered flying Drosophila.. Learn. Mem. 4: 318–327. [Abstract]
Wolf, R. and Heisenberg, M. 1998. Fifth International Congress of Neuroethology, San Diego, CA.
Wolf, R., Voss, A., Hein, S., Heisenberg, M. 1992. Can a fly ride a bicycle? Discussion on natural and artificial low-level seeing systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 337: 261–269.
Wolf, R., Wittig, T., Liu, L., Wustmann, G., Eyding, D., Heisenberg, M. 1998. Drosophila mushroom bodies are dispensable for visual, tactile and motor learning. Learn. Mem. 5: 166–178.
Wyszecki, G. and Stiles, W.S. In Color science—concepts and methods, quantitative data and formulae .1982. Wiley, New York.
Xia, S.Z., Liu, L., Feng, C.H., Guo, A. 1997a. Memory consolidation in Drosophila operant visual learning. Learn. Mem. 4: 205–218. [Abstract]
Xia, S.Z., Liu, L., Feng, C.H., Guo, A.K. 1997b. Nutritional effects on operant visual learning in Drosophila melanogaster.. Physiol. Behav. 62: 263–271. [CrossRef][Medline]
Xia, S.Z., Feng, C.H., Guo, A.K. 1999. Temporary amnesia induced by cold anesthesia and hypoxia in Drosophila.. Physiol. Behav. 65: 617–623. [Medline]