|
Full citation: Brembs B.; Baxter D.A. and Byrne J.H. (2004): Extending In Vitro Conditioning in Aplysia to Analyze Operant and Classical Processes in the Same Preparation. Learn. Mem. 11: 412-420. (PDF) |
Extending In Vitro Conditioning in Aplysia to Analyze Operant and Classical Processes in the Same Preparation Department of Neurobiology and Anatomy, W.M. Keck Center for the Neurobiology of Learning and Memory, The University of Texas Medical School at Houston, Houston, Texas 77030, USA |
| ABSTRACT |
|---|
|
|
|---|
Operant and classical conditioning are major processes shaping behavioral responses in all animals. Although the understanding of the mechanisms of classical conditioning has expanded significantly, the understanding of the mechanisms of operant conditioning is more limited. Recent developments in Aplysia are helping to narrow the gap in the level of understanding between operant and classical conditioning, and have raised the possibility of studying the neuronal processes underlying the interaction of operant and classical components in a relatively complex learning task. In the present study, we describe a first step toward realizing this goal, by developing a single in vitro preparation in which both operant and classical conditioning can be studied concurrently. The new paradigm reproduced previously published results, even under more conservative and homogenous selection criteria and tonic stimulation regime. Moreover, the observed learning was resistant to delay, shortening, and signaling of reinforcement.
Introduction
Ambulatory animals continuously face changing environmental situations.
However, not all events are random occurrences. Some events are direct
consequences either of the behavior of the animal or of some other events in
the environment. If the nonrandom events are significant, animals that can
predict them will have a strong adaptive advantage. Some of the most regular
predictive relationships are inborn (e.g., reflexes), but many others are
learned. Operant or instrumental conditioning is a form of learning in which
an animal learns the predictive relationship between behaviors and the
environment (Thorndike 1911![]() ;
Skinner 1938
;
Skinner 1938![]() ), whereas
classical or Pavlovian conditioning is a form of learning in which an animal
learns the relationship between two environmental events
(Pavlov 1927
), whereas
classical or Pavlovian conditioning is a form of learning in which an animal
learns the relationship between two environmental events
(Pavlov 1927![]() ). In freely
moving animals in the wild, it can be difficult to distinguish between the
two, because a feedback loop exists between the behavior of the animal and the
environment. For example, a frog may discover a small moving object while
foraging for prey, extend its tongue toward the object, find that the object
is striped and produces a noxious sting and hence in the future avoid striped
insects. This well-known example of aversive conditioning illustrates the
feedback loop between behavior and stimuli. The foraging behavior led to the
perception of the moving object, which in turn elicited the extension of the
tongue, which in turn had the noxious sting as a consequence, which in turn
led to the avoidance of striped insects by the frog. It is not clear a priori
which events have been remembered by the frog. Clearly, the stripes were
somehow associated with the sting (a classical association between two
stimuli), but was the extension of the tongue instrumental in this
association? To understand such interacting events, it is necessary to first
reduce them to their operant and classical components and then join them again
under controlled conditions.
). In freely
moving animals in the wild, it can be difficult to distinguish between the
two, because a feedback loop exists between the behavior of the animal and the
environment. For example, a frog may discover a small moving object while
foraging for prey, extend its tongue toward the object, find that the object
is striped and produces a noxious sting and hence in the future avoid striped
insects. This well-known example of aversive conditioning illustrates the
feedback loop between behavior and stimuli. The foraging behavior led to the
perception of the moving object, which in turn elicited the extension of the
tongue, which in turn had the noxious sting as a consequence, which in turn
led to the avoidance of striped insects by the frog. It is not clear a priori
which events have been remembered by the frog. Clearly, the stripes were
somehow associated with the sting (a classical association between two
stimuli), but was the extension of the tongue instrumental in this
association? To understand such interacting events, it is necessary to first
reduce them to their operant and classical components and then join them again
under controlled conditions.
Laboratory studies of classical conditioning have successfully interrupted
the operant-classical feedback loop such that the behavior of the animal is
irrelevant and the two environmental events (the conditioned stimulus, CS,
which predicts the unconditioned stimulus, US) can be traced from their
sensory afferents to the brain and, finally, to the point where they converge
and the learning occurs (e.g., Walters and
Byrne 1983![]() ; Bao et al.
1998
; Bao et al.
1998![]() ; Hawkins et al.
1998
; Hawkins et al.
1998![]() ; Kim et al.
1998
; Kim et al.
1998![]() ; Lechner et al.
2000a
; Lechner et al.
2000a![]() ,b
,b![]() ;
Schafe et al. 2001
;
Schafe et al. 2001![]() ;
Medina et al. 2002
;
Medina et al. 2002![]() ;
Paschall and Davis 2002
;
Paschall and Davis 2002![]() ;
Ressler et al. 2002
;
Ressler et al. 2002![]() ;
Antonov et al. 2003
;
Antonov et al. 2003![]() ;
Crow and Tian 2003
;
Crow and Tian 2003![]() ;
Davis et al. 2003
;
Davis et al. 2003![]() ;
Epstein et al. 2003
;
Epstein et al. 2003![]() ;
Flynn et al. 2003
;
Flynn et al. 2003![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ;
Nader 2003
;
Nader 2003![]() ). An analogous
convergence point between operant behavior and the unconditioned stimulus (or
reinforcer in the operant nomenclature) has recently been described in
Aplysia (Nargeot et al.
1999a
). An analogous
convergence point between operant behavior and the unconditioned stimulus (or
reinforcer in the operant nomenclature) has recently been described in
Aplysia (Nargeot et al.
1999a![]() ,b
,b![]() ;
Brembs et al. 2002
;
Brembs et al. 2002![]() ).
).
The carefully controlled operant and classical conditioning protocols used
in laboratory studies are somewhat artificial learning situations, because the
closed feedback loop between behavioral outputs and sensory inputs in a freely
moving animal inevitably leads to many sensory stimuli eliciting behavioral
responses and many behavioral actions causing the perception of sensory
stimuli, all at or near the same time. One would expect that evolutionary
selection pressures would form around the natural situation in which both
operant and classical predictors play their parts simultaneously, so that this
situation may be more easily learned than in the separate, experimental cases
(i.e., composite conditioning; Brembs
2000![]() ; Brembs and Heisenberg
2000
; Brembs and Heisenberg
2000![]() ; Heisenberg et al.
2001
; Heisenberg et al.
2001![]() ). On the other hand, studies from vertebrates suggest that
such a combination can have various effects, depending on subtle details
(Williams 1975
). On the other hand, studies from vertebrates suggest that
such a combination can have various effects, depending on subtle details
(Williams 1975![]() ;
Williams and Heyneman 1982
;
Williams and Heyneman 1982![]() ;
Williams 1989
;
Williams 1989![]() ;
Williams et al. 1990
;
Williams et al. 1990![]() ;
Hammerl 1993
;
Hammerl 1993![]() ; Reed
1996
; Reed
1996![]() ,
1999
,
1999![]() ,
2003
,
2003![]() ;
Williams 1999
;
Williams 1999![]() ). Therefore, as
a first step toward studying the neurobiological underpinnings of operant and
classical interactions, we have designed an experimental system in which
operant and classical conditioning can be investigated separately,
concurrently, or sequentially and which is amenable to cellular and network
analysis. We took advantage of the recent advances in operant and classical
conditioning of Aplysia feeding behavior
(Susswein and Schwarz 1983
). Therefore, as
a first step toward studying the neurobiological underpinnings of operant and
classical interactions, we have designed an experimental system in which
operant and classical conditioning can be investigated separately,
concurrently, or sequentially and which is amenable to cellular and network
analysis. We took advantage of the recent advances in operant and classical
conditioning of Aplysia feeding behavior
(Susswein and Schwarz 1983![]() ;
Schwarz and Susswein 1986
;
Schwarz and Susswein 1986![]() ;
Colwill et al. 1997
;
Colwill et al. 1997![]() ; Nargeot et
al. 1997
; Nargeot et
al. 1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() ;
Lechner et al.
2000a
;
Lechner et al.
2000a![]() ,b
,b![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ) and
developed a computer-supported, single Aplysia preparation in which
operant and classical experiments can be conducted both separately and in
combination.
) and
developed a computer-supported, single Aplysia preparation in which
operant and classical experiments can be conducted both separately and in
combination.
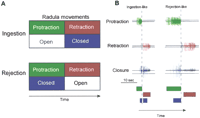
The feeding behavior of Aplysia
(Fig. 1) offers a useful system
in which to investigate classical and operant conditioning. Recently,
substantial progress has been made toward understanding the neurobiology of
operant conditioning of feeding behavior in Aplysia (Nargeot et al.
1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() ;
Brembs et al. 2002
;
Brembs et al. 2002![]() ;
Katzoff et al. 2002
;
Katzoff et al. 2002![]() ) as well
as toward understanding the neurobiology of classical conditioning (Lechner et
al.
2000a
) as well
as toward understanding the neurobiology of classical conditioning (Lechner et
al.
2000a![]() ,b
,b![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ).
).
Given the greater accessibility for neurobiological research, we chose to
work in vitro, with reduced preparations of the Aplysia CNS, similar
to the two previously developed in our laboratory. One in vitro preparation
has been developed to study operant conditioning and another to study
classical conditioning (Nargeot et al.
1997![]() ; Mozzachiodi et al.
2003
; Mozzachiodi et al.
2003![]() ). These preparations are rather similar. For example, in
both, patterned motor outputs (buccal motor patterns, BMPs) are recorded
extracellularly from the peripheral nerves of the buccal ganglia. This
patterned activity can be interpreted as the commands for the movements of the
radula/odontophore (a tongue-like organ), which lead to ingestion (or
rejection) behavior (i.e., fictive feeding behavior,
Fig. 1). Ingestion behavior can
be classically and operantly conditioned in vivo
(Susswein et al. 1983
). These preparations are rather similar. For example, in
both, patterned motor outputs (buccal motor patterns, BMPs) are recorded
extracellularly from the peripheral nerves of the buccal ganglia. This
patterned activity can be interpreted as the commands for the movements of the
radula/odontophore (a tongue-like organ), which lead to ingestion (or
rejection) behavior (i.e., fictive feeding behavior,
Fig. 1). Ingestion behavior can
be classically and operantly conditioned in vivo
(Susswein et al. 1983![]() ;
Susswein et al. 1986
;
Susswein et al. 1986![]() ;
Lechner et al. 2000b
;
Lechner et al. 2000b![]() ;
Brembs et al. 2002
;
Brembs et al. 2002![]() ). The
esophageal nerve (En2) conveys the US
(Schwarz and Susswein 1986
). The
esophageal nerve (En2) conveys the US
(Schwarz and Susswein 1986![]() ;
Nargeot et al. 1997
;
Nargeot et al. 1997![]() ;
Lechner et al. 2000b
;
Lechner et al. 2000b![]() ;
Brembs et al. 2002
;
Brembs et al. 2002![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ) and
the anterior tentacle nerve (AT4) conveys the CS (Lechner et al.
2000a
) and
the anterior tentacle nerve (AT4) conveys the CS (Lechner et al.
2000a![]() ,b
,b![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ). In
the analog of classical conditioning the CS and US are delivered as electrical
stimulation of these nerves. Thus, in behavioral terms, the BMPs constitute
the operant behavior (ingestion or rejection; Morton and Chiel
1993a
). In
the analog of classical conditioning the CS and US are delivered as electrical
stimulation of these nerves. Thus, in behavioral terms, the BMPs constitute
the operant behavior (ingestion or rejection; Morton and Chiel
1993a![]() ,b
,b![]() ;
Nargeot et al. 1997
;
Nargeot et al. 1997![]() ) and
extracellular stimulations of the aforementioned nerves constitute the
environmental feedback (i.e., stimulation of AT4 simulates tactile
stimulation of the lips; Lechner et al.
2000a
) and
extracellular stimulations of the aforementioned nerves constitute the
environmental feedback (i.e., stimulation of AT4 simulates tactile
stimulation of the lips; Lechner et al.
2000a![]() ,b
,b![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ;
stimulation of En2 simulates food reward,
Brembs et al. 2002
;
stimulation of En2 simulates food reward,
Brembs et al. 2002![]() ).
).
However, besides the training protocol (operant vs. classical), there is
one major difference between the two preparations. The preparation for
classical conditioning included the cerebral ganglion, because it mediates the
CS pathway (Lechner et al.
2000a![]() ,b
,b![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ),
whereas the operant procedure did not (Nargeot et al.
1997
),
whereas the operant procedure did not (Nargeot et al.
1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() ).
).
Thus, to be able to study the interaction of operant and classical conditioning, we developed a single buccal/cerebral preparation in which classical and operant conditioning experiments can be conducted and the results compared. Moreover, this preparation will allow for the concurrent presentation of classical and operant predictors, and thereby provide a preparation that is suitable for cellular analyses of composite learning. As part of this study, we also developed a computer-assisted neuronal pattern recognition system to identify the BMPs. Most stimulation parameters were entirely computer controlled. The new preparation reproduced the previously published operant learning. Various parameter modifications indicated that the in vitro conditioning was rather robust.
| RESULTS |
|---|
|
|
|---|
The first step toward developing a preparation in which the interaction of
classical conditioning and operant conditioning can be analyzed was to
determine whether in vitro operant conditioning is expressed in the
preparation originally developed to study classical conditioning
(Mozzachiodi et al. 2003![]() ).
Specifically, we subjected a preparation consisting of the isolated cerebral
ganglion and buccal ganglion to the in vitro protocol of Nargeot et al.
(1997
).
Specifically, we subjected a preparation consisting of the isolated cerebral
ganglion and buccal ganglion to the in vitro protocol of Nargeot et al.
(1997![]() ) and investigated the
extent to which the preparation reproduced the previous results. The cerebral
ganglion contains higher-order neurons that can trigger the occurrence of BMPs
in the buccal ganglia (Rosen et al.
1991
) and investigated the
extent to which the preparation reproduced the previous results. The cerebral
ganglion contains higher-order neurons that can trigger the occurrence of BMPs
in the buccal ganglia (Rosen et al.
1991![]() ; Jing and Weiss
2001
; Jing and Weiss
2001![]() ,
2002
,
2002![]() ;
Hurwitz et al. 2003
;
Hurwitz et al. 2003![]() ). It is
unknown whether it also contains cells that can silence neural activity in the
buccal ganglia. Although preliminary experiments, in which we recorded from
the cerebral-to-buccal connective (CBC) during spontaneous BMPs, did not
reveal any evidence that spontaneous BMPs are either elicited or suppressed by
signals originating in the cerebral ganglion (data not shown), the presence of
either type of cell could disrupt either the occurrence of spontaneous BMPs,
the ability of BMPs to be conditioned, or both.
). It is
unknown whether it also contains cells that can silence neural activity in the
buccal ganglia. Although preliminary experiments, in which we recorded from
the cerebral-to-buccal connective (CBC) during spontaneous BMPs, did not
reveal any evidence that spontaneous BMPs are either elicited or suppressed by
signals originating in the cerebral ganglion (data not shown), the presence of
either type of cell could disrupt either the occurrence of spontaneous BMPs,
the ability of BMPs to be conditioned, or both.
As part of the study, we also developed a computer program (see Materials and Methods) that allowed for the control of the stimulation schedule and parameters, and to assist in distinguishing between the different types of patterns and therefore eliminate the need for a blind observer. A final aspect of the study was to vary the stimulation parameters to investigate the feasibility of experiments in which operant and classical predictors are combined.
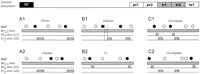
All preparations were treated identically up until the start of the
experiment, where each preparation was randomly assigned to one of six groups
(Fig. 2A,B,C). These groups
were designed as two triplets, the difference between the two being that one
received contingent reinforcement via stimulation of the esophageal nerve and
the other did not (see Materials and Methods for details;
Fig. 2A,B,C). Note that some of
the noncontingent groups received contingent CSs, but never contingent USs.
The groups were all operant in nature and received tonic Bn2,3
stimulation throughout the experiment. This nerve provides afferent input to
the buccal ganglia. Stimulation of Bn2,3 at a constant rate with
weak intensity stimuli increases the likelihood of generating spontaneous BMPs
(Nargeot et al. 1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() ;
Fig. 2A,B,C). Only
ingestion-like BMPs (iBMPs) were reinforced.
;
Fig. 2A,B,C). Only
ingestion-like BMPs (iBMPs) were reinforced.
|
Experimental Groups
The respective first groups in each triplet
(Fig. 2A) can be seen as
forming a pair designed to replicate previous studies of in vitro operant
conditioning (Nargeot et al.
1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ),
with minor parameter variations. It included a group that received a
contingent US (Fig. 2A1, UScon)
and a yoked control group (Fig.
2A2, USyoke). The expected outcome was an elevated number of iBMPs
in the contingently reinforced compared to the yoked control group.
),
with minor parameter variations. It included a group that received a
contingent US (Fig. 2A1, UScon)
and a yoked control group (Fig.
2A2, USyoke). The expected outcome was an elevated number of iBMPs
in the contingently reinforced compared to the yoked control group.
The respective second groups (Fig. 2B) were designed to test the effect of a delay and shortening of the reinforcing stimulus (US), as well as the effect of adding a contingent CS without a US. The contingently reinforced group (USdcon, Fig. 2B1) received a contingent US as the "UScon" group. But compared to the UScon group, the US was shortened from 6 to 4 sec and delayed by 2 sec (USdcon, Fig. 2B1). The other group (CS, Fig. 2B2) received only contingent CS presentations and no US presentations. This group was included to control for possible effects of contingent CSs alone (Fig. 2B2). The expected outcome was an elevated number of iBMPs in the USdcon group versus any of the noncontingent groups, and an unaffected number of BMPs in the group that only received a CS, compared to the other two noncontingent groups (i.e., Figs. 2A2, 1C2). Potentially, the USdcon group could have shown a lower number of BMPs than either the UScon (Fig. 2A1) or the CS+USdcon (Fig. 2C1) group.
The respective last groups in each triplet (Fig. 2C) were designed to investigate the effect of combining the shortened and delayed US with a contingent CS to "signal" the occurrence of the US (Fig. 2C). Both groups received contingent CS presentations after every iBMP, throughout the experiment. The contingently reinforced group (CS+USdcon) received contingent US presentations after each iBMP/CS combination (Fig. 2C1), whereas the control group (CS+USyoke) received the same sequence of US presentations as the contingently reinforced group, but independent of its behavior (yoked control; Fig. 2C2). In an intact Aplysia, the protocol of CS+USdcon would be analogous to a bite (iBMP) leading to a tactile stimulation of the lips (AT4 stimulation) followed by food (En2 stimulation).
Thus, in the contingently reinforced group
(Fig. 2C1; CS+USdcon), during
training the CS signaled the occurrence of reinforcement (US), whereas in the
yoked control group (Fig. 2C2;
CS+USyoke) it did not. The expected outcome is a higher number of BMPs in the
contingently reinforced as compared to the yoked control. In vertebrates, such
signaling can increase or decrease the amount of operant responding, depending
on the choice of parameters (Williams
1975![]() ; Williams and Heyneman
1982
; Williams and Heyneman
1982![]() ; Williams
1989
; Williams
1989![]() ; Williams et al.
1990
; Williams et al.
1990![]() ; Hammerl
1993
; Hammerl
1993![]() ; Reed 1996
; Reed 1996![]() ,
1999
,
1999![]() ,
2003
,
2003![]() ;
Williams 1999
;
Williams 1999![]() ). If a signaling
effect of the CS is present in the preparation, the number of iBMPs in the
CS+USdcon group is expected to be higher or lower than the number of BMPs in
either the UScon or the USdcon group.
). If a signaling
effect of the CS is present in the preparation, the number of iBMPs in the
CS+USdcon group is expected to be higher or lower than the number of BMPs in
either the UScon or the USdcon group.
BMP Analysis
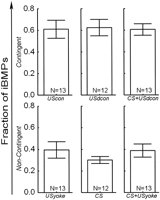
In order to assess the effects of the different treatments on the buccal Central Pattern Generator, three levels of analysis were used. First, we analyzed the total number of BMPs, irrespective of BMP-type. To gather more detailed information, we then analyzed the fraction of BMPs that were ingestion-like in nature (i.e., iBMPs). This measure has the advantage in that it describes the propensity of a preparation to produce iBMPs, irrespective of the total number of patterns produced. Finally, we evaluated the absolute number of iBMPs versus all other BMPs, to gain insight into the absolute changes in the generation of BMPs.
A one-way ANOVA (see Materials and Methods) over the total number of BMPs in all six groups did not reveal any significant variations in the total number of BMPs produced, neither in the pretest period immediately preceding the training (SS = 41.5, DF = 5, MS = 8.3, F = 0.48, p = 0.8), nor in the test immediately after the training (SS = 38.1, DF = 5, MS = 7.6, F = 0.38, p = 0.9). Thus, groups did not differ in their propensity to produce BMPs, before or after the training (i.e., treatment did not have any effect on the total number of all BMPs produced by the preparations).
Next, the fraction of iBMPs was evaluated. A one-way ANOVA over the six groups in the pretest period immediately preceding the training, was not significant (SS = 0.18; DF = 5; MS = 0.036; F = 0.74; p = 0.6). Thus, the six different groups did not differ significantly in the fraction of iBMPs produced before the training. This result indicates that all preparations had the same propensity to produce ingestion-like BMPs and any difference after training can only be attributed to the parameters of the stimulations during training.
All Contingently Reinforced Groups Increased the Propensity to Produce iBMPs
A one way ANOVA over the fraction of iBMPs in the six groups in the five minutes immediately following training, was significant (SS = 1.26; DF = 5; MS = 0.25; F = 4.5; p = 0.001). Fisher LSD post-hoc tests reveal that this significance was due to only the contingently reinforced groups differing from all noncontingent groups (Table 1). Thus, none of the different variations in US timing and duration had any effect on the magnitude of learning: contingently reinforced (via stimulation of En2) preparations produced on average a larger fraction of iBMPs than preparations that received either no US at all or noncontingent USs, irrespective of the US parameters (Fig. 3).
|
|
Stimulation of the AT4 nerve (such as the CS used here) can also
elicit iBMPs, either after classical conditioning
(Lechner et al. 2000a![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ) or if
the stimulation is sufficiently intense. The application of contingent CSs in
our experiments seemed to decrease (albeit insignificantly) the number of
iBMPs (see Fig. 3; CS). To
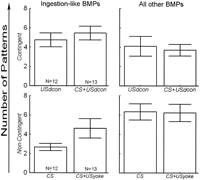
assess whether there was any effect from the presence or absence of the
inserted CS, signaling the US, which was not uncovered by evaluating the
fraction of iBMPs, a two-way repeated measures ANOVA was carried out over the
absolute number of iBMPs and all other BMPs
(Fig. 4). The first factor
tested between contingently reinforced and noncontingent groups, whereas the
second tested between pairs, and the repeated measures factor tested for
differences between iBMPs and all other BMPs
(Fig. 4). Only the groups with
comparable US duration (4 sec) were compared, because these groups differed
only in the presence or absence of the CS. Only the interaction between the
repeated measures factor and the experimental/control factor was significant
(SS = 92.9, DF = 1, F = 15.0, p = 0.0003;
Fig. 4), meaning the
distinction between experimental and control groups (i.e., the training
regime) had a significant effect on the distribution of iBMPs and other BMPs
among the groups. This result indicates that the presence or absence of the CS
did not, but only the presence or absence of a contingency between
ingestion-like BMPs and the US did have a statistically verifiable effect on
the types of BMPs that were produced in the different groups. Thus, with our
stimulation parameters, AT4 stimulation by itself had no direct
operant effects. The result corroborates our conclusions from the analysis of
the fraction of iBMPs, namely that contingent reinforcement increases the
relative number of iBMPs. In addition, a Fisher's LSD post-hoc analysis
revealed that in the control groups, less ingestion-like BMPs are produced
than other BMPs (p < 0.001) and that the number of other BMPs in the
experimental groups is reduced, compared to the control groups (p < 0.01).
Presumably because of the high value in the CS+USyoke group, the comparison of
ingestion-like BMPs in experimental versus control groups fails to reach
statistical significance (p < 0.12: see Discussion).
) or if
the stimulation is sufficiently intense. The application of contingent CSs in
our experiments seemed to decrease (albeit insignificantly) the number of
iBMPs (see Fig. 3; CS). To
assess whether there was any effect from the presence or absence of the
inserted CS, signaling the US, which was not uncovered by evaluating the
fraction of iBMPs, a two-way repeated measures ANOVA was carried out over the
absolute number of iBMPs and all other BMPs
(Fig. 4). The first factor
tested between contingently reinforced and noncontingent groups, whereas the
second tested between pairs, and the repeated measures factor tested for
differences between iBMPs and all other BMPs
(Fig. 4). Only the groups with
comparable US duration (4 sec) were compared, because these groups differed
only in the presence or absence of the CS. Only the interaction between the
repeated measures factor and the experimental/control factor was significant
(SS = 92.9, DF = 1, F = 15.0, p = 0.0003;
Fig. 4), meaning the
distinction between experimental and control groups (i.e., the training
regime) had a significant effect on the distribution of iBMPs and other BMPs
among the groups. This result indicates that the presence or absence of the CS
did not, but only the presence or absence of a contingency between
ingestion-like BMPs and the US did have a statistically verifiable effect on
the types of BMPs that were produced in the different groups. Thus, with our
stimulation parameters, AT4 stimulation by itself had no direct
operant effects. The result corroborates our conclusions from the analysis of
the fraction of iBMPs, namely that contingent reinforcement increases the
relative number of iBMPs. In addition, a Fisher's LSD post-hoc analysis
revealed that in the control groups, less ingestion-like BMPs are produced
than other BMPs (p < 0.001) and that the number of other BMPs in the
experimental groups is reduced, compared to the control groups (p < 0.01).
Presumably because of the high value in the CS+USyoke group, the comparison of
ingestion-like BMPs in experimental versus control groups fails to reach
statistical significance (p < 0.12: see Discussion).
|
The limited number of preparations precludes statistically significant post-hoc differentiation between USdcon, CS+USyoke and CS+USdcon.
| DISCUSSION |
|---|
|
|
|---|
We developed a computer-assisted paradigm for in vitro operant and classical conditioning in Aplysia that included the isolated cerebral and buccal ganglia. As a first step we investigated whether the new preparation could exhibit operant conditioning and the robustness of the operant conditioning protocol to parameter variations including the presence of a CS signaling the reinforcer. The new paradigm reproduced previously published results, even under more conservative and homogenous selection criteria and tonic stimulation regime. Moreover, the observed learning was resistant to delay, shortening and signaling of reinforcement.
In Vitro Operant Conditioning Is Expressed in the Presence of the Cerebral Ganglion
The previous in vitro analog of operant conditioning consisted of only the
isolated buccal ganglia (Nargeot et al.
1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() ).
It was therefore necessary to replicate the finding in a more physiological
system that included the cerebral ganglion. The cerebral ganglion sends many
projections to the buccal ganglion and vice versa
(Rosen et al. 1991
).
It was therefore necessary to replicate the finding in a more physiological
system that included the cerebral ganglion. The cerebral ganglion sends many
projections to the buccal ganglion and vice versa
(Rosen et al. 1991![]() ; Jing and
Weiss 2001
; Jing and
Weiss 2001![]() ,
2002
,
2002![]() ;
Hurwitz et al. 2003
;
Hurwitz et al. 2003![]() ).
Therefore it was possible that the features of in vitro operant conditioning
may be fundamentally different with the cerebral ganglion attached. With one
exception (see below), we found that the features of operant conditioning were
remarkably similar to that obtained with only the buccal ganglion. Indeed,
after the six different training procedures, each contingently reinforced
group produced a larger percentage of iBMPs than each group that did not
receive contingent USs (Table
1; Fig. 3). Thus,
we have successfully extended the in vitro operant conditioning procedure
developed by Nargeot et al. (Nargeot et al.,
1997
).
Therefore it was possible that the features of in vitro operant conditioning
may be fundamentally different with the cerebral ganglion attached. With one
exception (see below), we found that the features of operant conditioning were
remarkably similar to that obtained with only the buccal ganglion. Indeed,
after the six different training procedures, each contingently reinforced
group produced a larger percentage of iBMPs than each group that did not
receive contingent USs (Table
1; Fig. 3). Thus,
we have successfully extended the in vitro operant conditioning procedure
developed by Nargeot et al. (Nargeot et al.,
1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() )
to include the connected cerebral ganglion.
)
to include the connected cerebral ganglion.
In Vitro Operant Conditioning With the Cerebral Ganglion is a Robust Phenomenon
We found that shortening and delaying the reinforcement by 2 sec did not disrupt the operant learning. We further found that adding a 2-sec CS between the ingestion-like BMPs and the reinforcement (US) also neither increased nor decreased the operant behavior.
Interestingly, delayed reinforcement is known from vertebrates to generally
decrease the rate at which the operant behavior controlling the reinforcement
is produced (e.g., Williams et al.
1990![]() ; Reed
1992a
; Reed
1992a![]() ,b
,b![]() ).
In the case of in vitro operant conditioning of Aplysia feeding
behavior this decrement due to delayed reinforcement apparently does not occur
within the range of parameters used in the present study. Clearly, a
sufficient delay of the US will eventually decrease the operant conditioning
effect, as will a further shortening of the US. Thus, our paradigm has
sufficient robustness to enable the study of US parameter variations: Slight
variations in the reinforcement schedule do not completely disrupt
learning.
).
In the case of in vitro operant conditioning of Aplysia feeding
behavior this decrement due to delayed reinforcement apparently does not occur
within the range of parameters used in the present study. Clearly, a
sufficient delay of the US will eventually decrease the operant conditioning
effect, as will a further shortening of the US. Thus, our paradigm has
sufficient robustness to enable the study of US parameter variations: Slight
variations in the reinforcement schedule do not completely disrupt
learning.
Importantly, the presentation of a sensory signal (or operant CS; the 2-sec
AT4 stimulation) of reinforcement in the delay after a BMP and
before reinforcement does not disrupt or enhance the production of
ingestion-like BMPs, compared to the situation in which the US is merely
delayed. This paradigm would be analogous to a behavior controlling both a
predictive neutral stimulus (the CS) and a biologically relevant one (the US)
at the same time. Returning to the example of a frog trying to capture a bee,
extending the tongue would lead to a sting (US) by the striped bee (CS). In an
intact Aplysia, the protocol would be analogous to a bite
(ingestion-like BMP) leading to a tactile stimulation of the lips
(AT4 stimulation) followed by food (En2 stimulation). It
is easy to assume that the tactile lip stimulus may be interpreted as the food
item moving, caused by the biting and swallowing movements. In both cases, the
operant (the tongue extension or the bite) and the classical (the stripes of
the bee or the lip stimulation) predictors can be perceived as competitors in
the animal's search for a predictor of the reinforcer
(Rescorla 1994![]() ) and antagonism
as well as synergism may result. The fact that our choice of parameters led to
neither synergism nor antagonism opens the possibility for parameter
variations that can generate these effects. For example, the delay between the
BMP and the US can be increased, allowing for a number of different
arrangements of the CS within that delay. Because AT4 stimulation
has been shown previously to be able to function as a predictive signal
(Mozzachiodi et al. 2003
) and antagonism
as well as synergism may result. The fact that our choice of parameters led to
neither synergism nor antagonism opens the possibility for parameter
variations that can generate these effects. For example, the delay between the
BMP and the US can be increased, allowing for a number of different
arrangements of the CS within that delay. Because AT4 stimulation
has been shown previously to be able to function as a predictive signal
(Mozzachiodi et al. 2003![]() ), the
optimal choice of parameters should be able to create increments and
decrements in the operant effect. The conspicuously high number of iBMPs in
the CS+USyoke group (Fig. 4) may be an indication of how such an effect may manifest itself. In some
preparations of the CS+USyoke group, concatenations of ingestion-like BMPs
were observed, caused by contingent CSs eliciting BMPs. Without the reduced
number of other BMPs in the CS+USyoke group and only the iBMPs thus enhanced,
it is tempting to interpret this as a nonassociative effect of a combination
of contingent CSs and noncontingent USs, particularly, since the CS+USdcon
group was the only other group where such a concatenation of BMP-CS-BMP was
observed. Although with our choice of parameters such effects were too weak to
reach statistical significance, it seems possible that a different set of
stimulation parameters could lead to a significant classical component in the
CS+USdcon group, which, in turn, would lead to all these preparations
exhibiting these concatenations of BMPs, while the yoked control preparations
would remain at the same level. The accessibility of the preparation allows
for a detailed analysis of the neuronal underpinnings of any such effects.
), the
optimal choice of parameters should be able to create increments and
decrements in the operant effect. The conspicuously high number of iBMPs in
the CS+USyoke group (Fig. 4) may be an indication of how such an effect may manifest itself. In some
preparations of the CS+USyoke group, concatenations of ingestion-like BMPs
were observed, caused by contingent CSs eliciting BMPs. Without the reduced
number of other BMPs in the CS+USyoke group and only the iBMPs thus enhanced,
it is tempting to interpret this as a nonassociative effect of a combination
of contingent CSs and noncontingent USs, particularly, since the CS+USdcon
group was the only other group where such a concatenation of BMP-CS-BMP was
observed. Although with our choice of parameters such effects were too weak to
reach statistical significance, it seems possible that a different set of
stimulation parameters could lead to a significant classical component in the
CS+USdcon group, which, in turn, would lead to all these preparations
exhibiting these concatenations of BMPs, while the yoked control preparations
would remain at the same level. The accessibility of the preparation allows
for a detailed analysis of the neuronal underpinnings of any such effects.
Thus, the operant effect described by Nargeot and colleagues is a robust, reproducible case of operant conditioning with the potential to study an even wider variety of behavior-CS-US relationships than space permits to present here.
Differences Between Previous Work
One of the results in Nargeot et al.
(1997![]() ) that could not be
reproduced was an increase in the total number of BMPs produced by the
contingently reinforced preparations. In our experiments the experimental
groups still produced more ingestion-like BMPs than the control groups, even
in absolute numbers (data not shown), but the most clear-cut results were
obtained when the frequency of ingestion-like BMPs was evaluated. Although we
would not exclude the possibility that this effect stems from the presence of
the cerebral ganglion, it could also be due to the asymmetrical selection
criteria that were used in Nargeot and colleagues' work. Nargeot and
colleagues discarded experimental preparations that produced less than five
ingestion-like BMPs during the 10-min training period. No such selection was
used for the control groups. Such a procedure may have selected animals in the
experimental group that showed an increase in general BMP activity,
independent of the operant conditioning. In our experiments, the same
selection criteria were used for both experimental and control groups (see
Materials and Methods). Because Nargeot and colleagues reinforced the first
ingestion-like BMP in each contingently reinforced preparation, there were no
latent inhibition effects that could have possibly reduced the ability of the
circuit to be conditioned. In our experiments, the amount of pretest was fixed
and any occurring ingestion-like BMPs during this time remained unreinforced.
Moreover, our selection regime required three ingestion-like BMPs from the
control groups as well and thus may have selected for too high a number of
ingestion-like BMPs in these groups, masking the effect of an increase in
total BMPs. Thus, while Nargeot and colleagues used a proactive selection
regime that may enhance any conditioning effects, our approach was more
conservative. Therefore, even under our testing conditions, the associative
conditioning effect found by Nargeot and colleagues could be reproduced,
emphasizing the robustness of the paradigm.
) that could not be
reproduced was an increase in the total number of BMPs produced by the
contingently reinforced preparations. In our experiments the experimental
groups still produced more ingestion-like BMPs than the control groups, even
in absolute numbers (data not shown), but the most clear-cut results were
obtained when the frequency of ingestion-like BMPs was evaluated. Although we
would not exclude the possibility that this effect stems from the presence of
the cerebral ganglion, it could also be due to the asymmetrical selection
criteria that were used in Nargeot and colleagues' work. Nargeot and
colleagues discarded experimental preparations that produced less than five
ingestion-like BMPs during the 10-min training period. No such selection was
used for the control groups. Such a procedure may have selected animals in the
experimental group that showed an increase in general BMP activity,
independent of the operant conditioning. In our experiments, the same
selection criteria were used for both experimental and control groups (see
Materials and Methods). Because Nargeot and colleagues reinforced the first
ingestion-like BMP in each contingently reinforced preparation, there were no
latent inhibition effects that could have possibly reduced the ability of the
circuit to be conditioned. In our experiments, the amount of pretest was fixed
and any occurring ingestion-like BMPs during this time remained unreinforced.
Moreover, our selection regime required three ingestion-like BMPs from the
control groups as well and thus may have selected for too high a number of
ingestion-like BMPs in these groups, masking the effect of an increase in
total BMPs. Thus, while Nargeot and colleagues used a proactive selection
regime that may enhance any conditioning effects, our approach was more
conservative. Therefore, even under our testing conditions, the associative
conditioning effect found by Nargeot and colleagues could be reproduced,
emphasizing the robustness of the paradigm.
Outlook
In the future, this in vitro operant/classical conditioning paradigm can be
employed to examine such long-standing questions as whether there are any
operant components even in purely classical conditioning (e.g.,
Gormezano and Tait 1976![]() and
references therein) or whether classical and operant conditioning are merely
two aspects of the same conditioning processes
(Skinner 1935
and
references therein) or whether classical and operant conditioning are merely
two aspects of the same conditioning processes
(Skinner 1935![]() ; Konorski and
Miller
1937a
; Konorski and
Miller
1937a![]() ,b
,b![]() ;
Skinner 1937
;
Skinner 1937![]() ;
Rescorla and Solomon 1967
;
Rescorla and Solomon 1967![]() ;
Trapold and Winokur 1967
;
Trapold and Winokur 1967![]() ;
Trapold et al. 1968
;
Trapold et al. 1968![]() ;
Trapold and Overmier 1972
;
Trapold and Overmier 1972![]() ;
Rescorla and Holland 1982
;
Rescorla and Holland 1982![]() ;
Rescorla
1990a
;
Rescorla
1990a![]() ,b
,b![]() ,
1994
,
1994![]() ;
Brembs and Heisenberg 2000
;
Brembs and Heisenberg 2000![]() ;
Heisenberg et al. 2001
;
Heisenberg et al. 2001![]() ;
Corbit et al. 2003
;
Corbit et al. 2003![]() ;
Holland and Gallagher 2003
;
Holland and Gallagher 2003![]() ;
Phillips et al. 2003
;
Phillips et al. 2003![]() ).
).
| MATERIALS AND METHODS |
|---|
|
|
|---|
General Methods
Aplysia californica (80-350 g) were obtained from Alacrity Marine
Biological Specimens and Marinus and housed individually in perforated plastic
cages, floating in aerated seawater tanks at 15°C. Animals were fed  1
g of dried seaweed three times a week. To help ensure that all animals were in
a similar motivational state, experimental animals were food deprived 3-5 d
before the dissection.
1
g of dried seaweed three times a week. To help ensure that all animals were in
a similar motivational state, experimental animals were food deprived 3-5 d
before the dissection.
Dissection
Prior to dissection, the motivational state of all animals was enhanced by
first feeding them a small piece of dried seaweed ( 1.5 cm2)
and 30 min later a larger (8-cm2) piece. While the animal was
feeding on the larger piece, it was anaesthetized by an injection of isotonic
MgCl2 equivalent to 50% of its body mass. The dissection follows
the procedure described in Nargeot et al.
(1997
1.5 cm2)
and 30 min later a larger (8-cm2) piece. While the animal was
feeding on the larger piece, it was anaesthetized by an injection of isotonic
MgCl2 equivalent to 50% of its body mass. The dissection follows
the procedure described in Nargeot et al.
(1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() ):
An incision was made along the midline of the foot to expose the buccal mass
and the esophagus. The most medial-ventral branch (designated branch 4) of the
right anterior tentacle nerve (AT, for nomenclature, see
Jahan-Parwar and Fredman
1976
):
An incision was made along the midline of the foot to expose the buccal mass
and the esophagus. The most medial-ventral branch (designated branch 4) of the
right anterior tentacle nerve (AT, for nomenclature, see
Jahan-Parwar and Fredman
1976![]() ), which terminates in the lip region of the animal, was
retained. All other peripheral nerves of the cerebral ganglion were cut short.
The esophagus and the buccal mass together with the cerebral and buccal
ganglia were removed and transferred to a chamber containing artificial
seawater with a high concentration of divalent cations (high divalent ASW)
composed of (in mM): NaCl 210, KCl 10, MgCl2 145, MgSO4
20, CaCl2 33, and HEPES 10 (pH adjusted to 7.5 with NaOH). The high
divalent ASW was used to decrease neural activity during further dissection
(Byrne et al. 1978
), which terminates in the lip region of the animal, was
retained. All other peripheral nerves of the cerebral ganglion were cut short.
The esophagus and the buccal mass together with the cerebral and buccal
ganglia were removed and transferred to a chamber containing artificial
seawater with a high concentration of divalent cations (high divalent ASW)
composed of (in mM): NaCl 210, KCl 10, MgCl2 145, MgSO4
20, CaCl2 33, and HEPES 10 (pH adjusted to 7.5 with NaOH). The high
divalent ASW was used to decrease neural activity during further dissection
(Byrne et al. 1978![]() ). Selected
peripheral nerves of the buccal ganglion were retained for extracellular
recording and stimulation. The cerebral and the buccal ganglia were then
pinned to the bottom of a petri dish coated with silicone elastomer (Sylgard,
Dow Corning). In all experiments, the connective tissue sheath that covers the
ganglia was left intact. The temperature of the static bath was maintained at
15°C with a feedback-controlled Peltier cooling device (Model SE 5010,
Marlow Industries). The high divalent ASW was exchanged for normal ASW for 30
min prior to the beginning of an experiment, once the extracellular electrodes
for both stimulation and recording were in place and tested for connectivity
(see below). The normal ASW was composed of (in mM): NaCl 450, KCl 10,
MgCl2 30, MgSO4 20, CaCl2 10, and HEPES 10
(pH adjusted to 7.5 with NaOH).
). Selected
peripheral nerves of the buccal ganglion were retained for extracellular
recording and stimulation. The cerebral and the buccal ganglia were then
pinned to the bottom of a petri dish coated with silicone elastomer (Sylgard,
Dow Corning). In all experiments, the connective tissue sheath that covers the
ganglia was left intact. The temperature of the static bath was maintained at
15°C with a feedback-controlled Peltier cooling device (Model SE 5010,
Marlow Industries). The high divalent ASW was exchanged for normal ASW for 30
min prior to the beginning of an experiment, once the extracellular electrodes
for both stimulation and recording were in place and tested for connectivity
(see below). The normal ASW was composed of (in mM): NaCl 450, KCl 10,
MgCl2 30, MgSO4 20, CaCl2 10, and HEPES 10
(pH adjusted to 7.5 with NaOH).
Extracellular Nerve Recordings
Previous in vivo recordings indicate that bursts of large-unit activity in
nerves I2n,Rn1 and Bn2,1 are associated with
the protraction, closure, and retraction, respectively, of the
radula/odontophore during feeding (Morton
and Chiel 1993b![]() ; Hurwitz et
al. 1996
; Hurwitz et
al. 1996![]() ). Moreover, in vitro recordings indicate that BMPs, which
represent fictive feeding, can be recorded from I2n,
Rn1, and Bn2,1
(Morton and Chiel 1993a
). Moreover, in vitro recordings indicate that BMPs, which
represent fictive feeding, can be recorded from I2n,
Rn1, and Bn2,1
(Morton and Chiel 1993a![]() ;
Nargeot et al. 1997
;
Nargeot et al. 1997![]() ;
Lechner et al. 2000a
;
Lechner et al. 2000a![]() ). Thus,
fictive feeding (i.e., BMPs) was monitored by placing silver electrodes on
nerves I2n,Rn1, and Bn2,1
(Nargeot et al. 1997
). Thus,
fictive feeding (i.e., BMPs) was monitored by placing silver electrodes on
nerves I2n,Rn1, and Bn2,1
(Nargeot et al. 1997![]() ) of the
right buccal ganglion (see below). All extracellular electrodes were isolated
from the surrounding bath using petroleum jelly (Vaseline, Sherwood Medical).
Signals were amplified with a differential AC amplifier (Model 1700, A-M
Systems). The amplified signals were displayed on a computer screen and saved
on the hard drive using a PCI 9112 A/D converter card (Adlink Technology,
Inc.) and custom-written software.
) of the
right buccal ganglion (see below). All extracellular electrodes were isolated
from the surrounding bath using petroleum jelly (Vaseline, Sherwood Medical).
Signals were amplified with a differential AC amplifier (Model 1700, A-M
Systems). The amplified signals were displayed on a computer screen and saved
on the hard drive using a PCI 9112 A/D converter card (Adlink Technology,
Inc.) and custom-written software.
Extracellular Nerve Stimulation
Similar to our previous studies (Nargeot et al.
1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() ;
Brembs et al. 2002
;
Brembs et al. 2002![]() ), electrical
stimulation (4-6 sec, 10 Hz, 0.5-msec pulses, 7 V) of the right
En2, which innervates the buccal mass
(Schwarz and Susswein, 1986
), electrical
stimulation (4-6 sec, 10 Hz, 0.5-msec pulses, 7 V) of the right
En2, which innervates the buccal mass
(Schwarz and Susswein, 1986![]() )
was used to mimic food reward. The duration and frequency of the stimulus
resembled bursts of activity recorded in vivo from En2 during
feeding (Brembs et al. 2002
)
was used to mimic food reward. The duration and frequency of the stimulus
resembled bursts of activity recorded in vivo from En2 during
feeding (Brembs et al. 2002![]() ).
En2 mediates several aspects of feeding behavior such as conveying
efferent activity that controls peristaltic movements of the gut
(Lloyd et al. 1988
).
En2 mediates several aspects of feeding behavior such as conveying
efferent activity that controls peristaltic movements of the gut
(Lloyd et al. 1988![]() ) and
conveying afferent activity that encodes information related to feeding
arousal (Susswein et al. 1984
) and
conveying afferent activity that encodes information related to feeding
arousal (Susswein et al. 1984![]() )
and satiety (Kuslansky et al.
1978
)
and satiety (Kuslansky et al.
1978![]() ,
1987
,
1987![]() ). Stimulation of
En2 has been used as a reinforcer to modify behavior and neural
activity in a training paradigm used for operant conditioning of
Aplysia feeding behavior both in vivo
(Brembs et al. 2002
). Stimulation of
En2 has been used as a reinforcer to modify behavior and neural
activity in a training paradigm used for operant conditioning of
Aplysia feeding behavior both in vivo
(Brembs et al. 2002![]() ) and in
vitro (Nargeot et al. 1997
) and in
vitro (Nargeot et al. 1997![]() )
and in classical conditioning (Mozzachiodi
et al. 2003
)
and in classical conditioning (Mozzachiodi
et al. 2003![]() ). Moreover, En2 is necessary for classical
conditioning of feeding behavior in vivo
(Lechner et al. 2000b
). Moreover, En2 is necessary for classical
conditioning of feeding behavior in vivo
(Lechner et al. 2000b![]() ).
Finally, En2 is necessary in an operant paradigm for learning that
food is inedible (Susswein and Schwarz
1983
).
Finally, En2 is necessary in an operant paradigm for learning that
food is inedible (Susswein and Schwarz
1983![]() ; Schwarz and Susswein
1986
; Schwarz and Susswein
1986![]() ). Thus, En2 appears to be part of the
reinforcement pathway that contributes to both classical and operant
conditioning.
). Thus, En2 appears to be part of the
reinforcement pathway that contributes to both classical and operant
conditioning.
Electrical stimulation of AT4 (2 sec, 5 Hz, 0.5-msec pulses) was
used to mimic the CS that was used in classical conditioning in vivo (Lechner
et al.
2000a![]() ,b
,b![]() )
and in vitro (Mozzachiodi et al.
2003
)
and in vitro (Mozzachiodi et al.
2003![]() ). The frequency of AT4 stimulation used in the
present study was similar to that recorded in vivo during mechanical
stimulation of the tentacles (Anderson
1967
). The frequency of AT4 stimulation used in the
present study was similar to that recorded in vivo during mechanical
stimulation of the tentacles (Anderson
1967![]() ; Fredman and Jahan-Parwar
1980
; Fredman and Jahan-Parwar
1980![]() ). The AT nerve mediates several aspects of feeding behavior.
For example, AT conveys afferent activity that encodes information about both
mechanical and chemical stimuli that signal the presence of food on the lips
(Anderson 1967
). The AT nerve mediates several aspects of feeding behavior.
For example, AT conveys afferent activity that encodes information about both
mechanical and chemical stimuli that signal the presence of food on the lips
(Anderson 1967![]() ;
Rosen et al. 1979
;
Rosen et al. 1979![]() ;
Xin et al. 1995
;
Xin et al. 1995![]() ). In addition,
AT conveys efferent activity that controls the movement of the lips
(Perrins and Weiss 1996
). In addition,
AT conveys efferent activity that controls the movement of the lips
(Perrins and Weiss 1996![]() ).
Several lines of evidence suggest that AT4 also mediates aspects of
the tactile CS that was used for in vivo classical conditioning (Lechner et
al.
2000a
).
Several lines of evidence suggest that AT4 also mediates aspects of
the tactile CS that was used for in vivo classical conditioning (Lechner et
al.
2000a![]() ,b
,b![]() ).
Finally, Lechner et al.
(2000a
).
Finally, Lechner et al.
(2000a![]() ) found that in vivo
classical conditioning (1) increased the probability that a weak stimulation
of AT4 would elicit BMPs, and (2) enhanced the
AT4-elicited synaptic input to B31/32 in cerebral and buccal
ganglia dissected from trained animals.
) found that in vivo
classical conditioning (1) increased the probability that a weak stimulation
of AT4 would elicit BMPs, and (2) enhanced the
AT4-elicited synaptic input to B31/32 in cerebral and buccal
ganglia dissected from trained animals.
Following Nargeot et al.
(1997![]() ), tonic stimulation of
the ventral branch of buccal nerve Bn2,3 (2 Hz, 0.5-msec pulses, 7
V) was used to nonspecifically elevate the number of spontaneous BMPs produced
by the preparation.
), tonic stimulation of
the ventral branch of buccal nerve Bn2,3 (2 Hz, 0.5-msec pulses, 7
V) was used to nonspecifically elevate the number of spontaneous BMPs produced
by the preparation.
Pulses for extracellular nerve stimulation were generated by a digital pulse generator (Pulsemaster A300, WPI) and applied, via a stimulus isolator (A360; WPI, Sarasota, FL), to bipolar silver electrodes that were placed on nerves Bn2,3, AT4, and En2 and isolated from the bath with Vaseline.
Once the extracellular electrodes were in place, the high divalent ASW was exchanged for normal ASW. Preparations were washed with 50 ml ASW and then single stimulations were applied to each of the three nerves to verify electrode connectivity. Pilot studies showed that due to the high incidence of BMPs immediately after the tonic stimulation of Bn2,3 was switched on, it was impossible to determine the appropriate sub-threshold AT4 intensity during Bn2,3 stimulation. Therefore, the intensity was empirically set to 3 V for all operant preparations, an intensity that on its own did not increase the number of BMPs in the pilot studies.
Classifications of BMPs
The feeding CPG expresses BMPs, which can be associated with ingestion or
rejection of food (Morton and Chiel
1993a![]() ,b
,b![]() ).
BMPs consist of specific patterns of neural activity, which correspond to
cycles of protraction and retraction of the radula/odontophore. BMPs can be
recorded from the buccal nerves I2n,Rn1, and
Bn2,1. Large-unit activity in I2n (i.e., radula
protraction) precedes large-unit activity in Bn2,1 (i.e., radula
retraction). Large-unit activity in Rn1 (i.e., radula closure)
overlaps to a varying extent with protraction and retraction activity
(Cropper et al. 1990
).
BMPs consist of specific patterns of neural activity, which correspond to
cycles of protraction and retraction of the radula/odontophore. BMPs can be
recorded from the buccal nerves I2n,Rn1, and
Bn2,1. Large-unit activity in I2n (i.e., radula
protraction) precedes large-unit activity in Bn2,1 (i.e., radula
retraction). Large-unit activity in Rn1 (i.e., radula closure)
overlaps to a varying extent with protraction and retraction activity
(Cropper et al. 1990![]() ; Morton
and Chiel
1993a
; Morton
and Chiel
1993a![]() ,b
,b![]() ;
Nargeot et al. 1997
;
Nargeot et al. 1997![]() ;
Kabotyanski et al. 2000
;
Kabotyanski et al. 2000![]() ). The
large-unit activity in Rn1 corresponds to action potentials in the
radula closure motor neuron B8, which has an axon in Rn1
(Morton and Chiel 1993b
). The
large-unit activity in Rn1 corresponds to action potentials in the
radula closure motor neuron B8, which has an axon in Rn1
(Morton and Chiel 1993b![]() ;
Nargeot et al. 1999b
;
Nargeot et al. 1999b![]() ).
).
As in previous studies (Morton and Chiel
1993a![]() ,b
,b![]() ;
Nargeot et al. 1997
;
Nargeot et al. 1997![]() ;
Lechner et al. 2000a
;
Lechner et al. 2000a![]() ; Jing and
Weiss 2001
; Jing and
Weiss 2001![]() ,
2002
,
2002![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ), we
classified BMPs as ingestion-like if
), we
classified BMPs as ingestion-like if 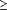 50% of radula closure
(Rn1) activity occurred after the termination of the protraction
(I2n) activity. The criterion for rejection-like BMPs was the
occurrence of closure (Rn1) activity during the protraction
(I2n) activity, but no overlap between closure (Rn1) and
retraction (Bn2,1) activity. BMPs that did not meet either of these
two criteria were classified as other BMPs
(Nargeot et al. 1997
50% of radula closure
(Rn1) activity occurred after the termination of the protraction
(I2n) activity. The criterion for rejection-like BMPs was the
occurrence of closure (Rn1) activity during the protraction
(I2n) activity, but no overlap between closure (Rn1) and
retraction (Bn2,1) activity. BMPs that did not meet either of these
two criteria were classified as other BMPs
(Nargeot et al. 1997![]() ;
Lechner et al. 2000a
;
Lechner et al. 2000a![]() ).
).
In the present study, only patterns that consisted of activity in all three buccal nerves clustered in a complete protraction/retraction cycle were classified as BMPs. Patterns consisting of bursts of activity in only one or two of the three nerves were classified as incomplete patterns and were not included in the study.
Computer-Assisted BMP Recognition
The custom-written software provided computer-assisted pattern recognition
(i.e., the computer attempted an online classification and suggested a pattern
type at the end of each BMP). The software was written on a MS Windows based
PC using C++ and the provided software development kit for the PCI 9112
converter card. The acquisition rate was limited by processor speed, in our
case to  8 kHz. The experimenter then determined whether to follow the
suggested classification or not. In the 30-min rest period, spontaneous BMPs
were used to individually adjust spike detection threshold and maximal
inter-spike-interval for each nerve to the individual BMPs of the experimental
animal. Using these two parameters, the computer then detected
"activity" in the three nerves (i.e., more than two spikes over
the threshold and within the given inter-spike-interval) and correlated the
timing of activity in the nerves according to the rules above. A colored line
along the baseline of the recordings denoted the detected pattern type. BMP
classification is usually unequivocal
(Nargeot et al. 1997
8 kHz. The experimenter then determined whether to follow the
suggested classification or not. In the 30-min rest period, spontaneous BMPs
were used to individually adjust spike detection threshold and maximal
inter-spike-interval for each nerve to the individual BMPs of the experimental
animal. Using these two parameters, the computer then detected
"activity" in the three nerves (i.e., more than two spikes over
the threshold and within the given inter-spike-interval) and correlated the
timing of activity in the nerves according to the rules above. A colored line
along the baseline of the recordings denoted the detected pattern type. BMP
classification is usually unequivocal
(Nargeot et al. 1997![]() ), but in
the few ambiguous cases where radula closure activity is divided almost
equally between protraction and retraction, the computer can make the
objective classification much faster than the human eye.
), but in
the few ambiguous cases where radula closure activity is divided almost
equally between protraction and retraction, the computer can make the
objective classification much faster than the human eye.
Procedures for In Vitro Training
The procedures were based on the in vitro operant conditioning experiment
developed by Nargeot et al.
(1997![]() ,
1999a
,
1999a![]() ,b
,b![]() ,c
,c![]() )
and on the in vitro classical conditioning procedure developed by Mozzachiodi
et al. (Lechner et al. 2000a
)
and on the in vitro classical conditioning procedure developed by Mozzachiodi
et al. (Lechner et al. 2000a![]() ;
Mozzachiodi et al. 2003
;
Mozzachiodi et al. 2003![]() ).
Unlike the cited operant experiments, our preparations were given a fixed
30-min rest period without any stimulation after the connectivity of all
electrodes was determined. After the rest period, two 5-min pretest periods
followed, which were followed immediately by two 5-min training periods,
similar to the in vivo experiments in Brembs et al.
(2002
).
Unlike the cited operant experiments, our preparations were given a fixed
30-min rest period without any stimulation after the connectivity of all
electrodes was determined. After the rest period, two 5-min pretest periods
followed, which were followed immediately by two 5-min training periods,
similar to the in vivo experiments in Brembs et al.
(2002![]() ). The experiment
concluded with a 5-min test period, which immediately followed training. USs
were only delivered to the preparation during training periods. Tonic
stimulation and, where applicable, CS delivery was performed throughout the
experiment. The CS presentation regime was kept constant throughout the
experiment, so that only the application of the US would differentiate between
training and test.
). The experiment
concluded with a 5-min test period, which immediately followed training. USs
were only delivered to the preparation during training periods. Tonic
stimulation and, where applicable, CS delivery was performed throughout the
experiment. The CS presentation regime was kept constant throughout the
experiment, so that only the application of the US would differentiate between
training and test.
Animals were divided randomly in six groups. Each group received tonic stimulation of Bn2,3, which began after the 30-min rest period and continued uninterrupted until the experiment ended. The groups differed from each other by the application regime of CS and US applications.
The first two groups were designed to replicate previous findings
(Nargeot et al. 1997![]() ) with the
difference that the cerebral ganglion was attached to the preparation. During
the training period, the UScon group received contingent reinforcement
(operant US deliveries) consisting of a 6-sec stimulation of En2
immediately following each ingestion-like BMP. The corresponding USyoke group
received the same sequence of En2 stimulations during training, but
uncorrelated with the occurrence of any BMPs ("yoked"
control).
) with the
difference that the cerebral ganglion was attached to the preparation. During
the training period, the UScon group received contingent reinforcement
(operant US deliveries) consisting of a 6-sec stimulation of En2
immediately following each ingestion-like BMP. The corresponding USyoke group
received the same sequence of En2 stimulations during training, but
uncorrelated with the occurrence of any BMPs ("yoked"
control).
The third group was designed to test for the effect of a delay and shortening of the US (USdcon). This group received a contingent 4-sec US with a 2-sec delay after each ingestion-like BMP produced during training.
The fourth group was designed to test the effect of introducing contingent CSs after each iBMP without a US. This group (CS) received contingent 2-sec AT4 stimulations (operant CSs) immediately after each ingestion-like BMP throughout the experiment and no USs during the training period.
The last two groups were designed to test the effects of introducing a signal of the delayed US. Both groups received contingent 2-sec AT4 stimulations (operant CSs) immediately after each ingestion-like BMP throughout the experiment, starting after the 30-min rest period. During training, the CS+USdcon group received contingent reinforcement (operant 4-sec USs) immediately upon cessation of the operant CS after each ingestion-like BMP. Thus, each ingestion-like BMP in this group was followed first by a CS and then by a US; both stimulations together yielded a total of 6 sec of stimulation after each ingestion-like BMP (the US in Nargeot and colleagues original experiment had been 6 sec as well). The CS+USyoke group received the same sequence of 4-sec En2 stimulations during the training period as the CS+USdcon group, but uncorrelated with either generated BMPs or received CSs (yoked control).
Preparations that did not produce at least one ingestion-like BMP during training and at least three ingestion-like BMPs in the entire experiment were discarded.
Statistics
One-way or multifactor Analyses of Variance (ANOVAs) were carried out to estimate the significance of within- and between-group differences. Fisher LSD Post-hoc tests were used to detect the significant contributions to the variance in the data.
| ACKNOWLEDGMENTS |
|---|
We thank R. Mozzachiodi for helpful comments on an earlier draft of the manuscript. Supported by an Emmy-Noether fellowship (B.B.) and NIH Research Grant R01 MH58423 (J.H.B.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
| FOOTNOTES |
|---|
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/content/full/11/4/412.
1 Present address: Institute for Neurobiology, Free University Berlin,
Königin-Luise-Straße 28/30, 14195 Berlin, Germany. ![]()
2 E-MAIL bjoern@brembs.net;FAX 49 308 385 5455.
| REFERENCES |
|---|
|
|
|---|
Anderson, J.A. 1967. Patterns of response of neurons in the cerebral ganglion of Aplysia californica. Exp. Neurol. 19:65-77.[Medline]
Antonov, I., Antonova, I., Kandel, E.R., and Hawkins, R.D.2003. Activity-dependent presynaptic facilitation and hebbian ltp are both required and interact during classical conditioning in Aplysia. Neuron37:135-147.[Medline]
Bao, J.X., Kandel, E.R., and Hawkins, R.D. 1998.
Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of
classical conditioning at Aplysia sensory-motor neuron synapses in
isolated cell culture. J. Neurosci.
18:458
-466.
Brembs, B. 2000. An analysis of associative conditioning in Drosophila at the flight simulator. Ph.D. thesis, University of Würzburg, Germany. http://opus.bibliothek.uni-wuerzburg.de/opus/volltexte/2002/103/pdf/diss.pdf
Brembs, B. and Heisenberg, M. 2000. The operant and
the classical in conditioned orientation in Drosophila melanogaster
at the flight simulator. Learn. Mem.
7: 104-115.
Brembs, B., Lorenzetti, F.D., Reyes, F.D., Baxter, D.A., and Byrne,
J.H. 2002. Operant reward learning in Aplysia: Neuronal
correlates and mechanisms. Science
296:1706
-1709.
Byrne, J.H., Castellucci, V.F., and Kandel, E.R. 1978.
Contribution of individual mechanoreceptor sensory neurons to defensive
gill-withdrawal reflex in Aplysia. J.
Neurophysiol. 41:418
-431.
Colwill, R., Goodrum, K., and Martin, A. 1997. Pavlovian appetitive discriminative conditioning in Aplysia californica. Anim Learn. Behav. 25:268 -276.
Corbit, L.H., Muir, J.L., and Balleine, B.W. 2003. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. Eur. J. Neurosci. 18:1286 -1294.[CrossRef][Medline]
Cropper, E.C., Kupfermann, I., and Weiss, K.R. 1990. Differential firing patterns of the peptide-containing cholinergic motor neurons b15 and b16 during feeding behavior in Aplysia. Brain Res. 522:176 -179.[CrossRef][Medline]
Crow, T. and Tian, L.M. 2003. Neural correlates of
Pavlovian conditioning in components of the neural network supporting ciliary
locomotion in Hermissenda. Learn. Mem.
10:209
-216.
Davis, M., Walker, D.L., and Myers, K.M. 2003. Role of
the amygdala in fear extinction measured with potentiated startle.
Ann. N.Y. Acad. Sci.
985:218
-232.
Epstein, H.T., Child, F.M., Kuzirian, A.M., and Alkon, D.L.2003 . Time windows for effects of protein synthesis inhibitors on Pavlovian conditioning in Hermissenda: Behavioral aspects. Neurobiol. Learn. Mem. 79:127 -131.[CrossRef][Medline]
Flynn, M., Cai, Y., Baxter, D.A., and Crow, T. 2003. A computational study of the role of spike broadening in synaptic facilitation of Hermissenda. J. Comput. Neurosci. 15: 29-41.[CrossRef][Medline]
Fredman, S.M. and Jahan-Parwar, B. 1980. Processing of chemosensory and mechanosensory information in identifiable Aplysia neurons. Comp. Biochem. Physiol. A 66: 25-34.[CrossRef]
Gormezano, I. and Tait, R.W. 1976. The Pavlovian analysis of instrumental conditioning. Pavlov. J. Biol. Sci. 11:37 -55.[Medline]
Hammerl, M. 1993. Blocking observed in human instrumental conditioning. Learn. Motiv. 24: 73-87.[CrossRef]
Hawkins, R.D., Greene, W., and Kandel, E.R. 1998. Classical conditioning, differential conditioning, and second-order conditioning of the Aplysia gill-withdrawal reflex in a simplified mantle organ preparation. Behav. Neurosci. 112:636 -645.[CrossRef][Medline]
Heisenberg, M., Wolf, R., and Brembs, B. 2001.
Flexibility in a single behavioral variable of Drosophila.
Learn. Mem. 8:1
-10.
Holland, P.C. and Gallagher, M. 2003. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 17:1680 -1694.[Medline]
Hurwitz, I., Neustadter, D., Morton, D.W., Chiel, H.J., and
Susswein, A.J. 1996. Activity patterns of the b31/b32 pattern
initiators innervating the i2 muscle of the buccal mass during normal feeding
movements in Aplysia californica. J.
Neurophysiol. 75:1309
-1326.
Hurwitz, I., Kupfermann, I., and Weiss, K.R. 2003.
Fast synaptic connections from cbis to pattern-generating neurons in
Aplysia: Initiation and modification of motor programs. J.
Neurophysiol. 89:2120
-2136.
Jahan-Parwar, B. and Fredman, S.M. 1976. Cerebral ganglion of Aplysia: Cellular organization and origin of nerves. Comp. Biochem. Physiol. A 54:347 -357.[Medline]
Jing, J. and Weiss, K.R. 2001. Neural mechanisms of
motor program switching in Aplysia. J.
Neurosci. 21:7349
-7362.
____. 2002. Interneuronal basis of the generation of
related but distinct motor programs in Aplysia: Implications for
current neuronal models of vertebrate intralimb coordination. J.
Neurosci. 22:6228
-6238.
Kabotyanski, E.A., Baxter, D.A., Cushman, S.J., and Byrne, J.H.2000
. Modulation of fictive feeding by dopamine and serotonin in
Aplysia. J. Neurophysiol.
83:374
-392.
Katzoff, A., Ben-Gedalya, T., and Susswein, A.J. 2002.
Nitric oxide is necessary for multiple memory processes after learning that a
food is inedible in Aplysia. J. Neurosci.
22:9581
-9594.
Kim, J.J., Krupa, D.J., and Thompson, R.F. 1998. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science 279:570 -573.
Konorski, J. and Miller, S. 1937a. On two types of conditioned reflex. J. Gen. Psychol. 16:264 -272.
____. 1937b. Further remarks on two types of conditioned reflex. J. Gen. Psychol. 17:405 -407.
Kuslansky, B., Weiss, K.R., and Kupfermann, I. 1978. A neural pathway mediating satiation of feeding behavior in Aplysia. Behav. Biol. 23:230 -237.[Medline]
Kuslansky, B., Weiss, K.R., and Kupfermann, I. 1987. Mechanisms underlying satiation of feeding behavior of the mollusc Aplysia. Behav. Neural Biol. 48:278 -303.[Medline]
Lechner, H.A., Baxter, D.A., and Byrne, J.H. 2000a.
Classical conditioning of feeding in Aplysia: II. Neurophysiological
correlates. J. Neurosci.
20:3377
-3386.
____. 2000b. Classical conditioning of feeding in
Aplysia: I. Behavioral analysis. J. Neurosci.
20:3369
-3376.
Lloyd, P.E., Kupfermann, I., and Weiss, K.R. 1988.
Central peptidergic neurons regulate gut motility in Aplysia.
J. Neurophysiol. 59:1613
-1626.
Medina, J.F., Christopher Repa, J., Mauk, M.D., and LeDoux, J.E.2002 . Parallels between cerebellum- and amygdala-dependent conditioning. Nat. Rev. Neurosci. 3: 122-131.[CrossRef][Medline]
Morton, D.W. and Chiel, H.J. 1993a. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J. Comp. Physiol. A 172:17 -32.[Medline]
____. 1993b. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J. Comp. Physiol. A 173:519 -536.[Medline]
Mozzachiodi, R., Lechner, H., Baxter, D., and Byrne, J.2003
. An in vitro analogue of classical conditioning of feeding
behavior in Aplysia. Learn. Mem.
10:478
-494.
Nader, K. 2003. Memory traces unbound. Trends Neurosci. 26:65 -72.[CrossRef][Medline]
Nargeot, R., Baxter, D.A., and Byrne, J.H. 1997.
Contingent-dependent enhancement of rhythmic motor patterns: An in vitro
analog of operant conditioning. J. Neurosci.
17:8093
-8105.
____. 1999a. In vitro analog of operant conditioning
in Aplysia. I. Contingent reinforcement modifies the functional
dynamics of an identified neuron. J. Neurosci.
19:2247
-2260.
____. 1999b. In vitro analog of operant conditioning
in Aplysia. II. Modifications of the functional dynamics of an
identified neuron contribute to motor pattern selection. J.
Neurosci. 19:2261
-2272.
____. 1999c. Dopaminergic synapses mediate neuronal
changes in an analogue of operant conditioning. J.
Neurophysiol. 81:1983
-1987.
Paschall, G.Y. and Davis, M. 2002. Second-order
olfactory-mediated fear-potentiated startle. Learn.
Mem. 9:395
-401.
Pavlov, I.P. 1927. Conditioned reflexes. Oxford University Press, Oxford, UK.
Perrins, R. and Weiss, K.R. 1996. A cerebral central
pattern generator in Aplysia and its connections with buccal feeding
circuitry. J. Neurosci.
16:7030
-7045.
Phillips, G.D., Setzu, E., Vugler, A., and Hitchcott, P.K.2003 . Immunohistochemical assessment of mesotelencephalic dopamine activity during the acquisition and expression of Pavlovian versus instrumental behaviours. Neuroscience 117:755 -767.[CrossRef][Medline]
Reed, P. 1992a. Effect of a signaled delay between an action and outcome on human judgment of causality. Q.J. Exp. Psychol. B 44B:81 -100.
____. 1992b. Signaled delay of reward—overshadowing versus sign-tracking explanations. Learn. Motiv. 23:27 -42.
____. 1996. Visual reinforcement signals interfere with the effects of reinforcer magnitude manipulations. Learn. Motiv. 27:464 -475.[CrossRef][Medline]
____. 1999. Role of a stimulus filling an action-outcome delay in human judgments of causal effectiveness. J. Exp. Psychol. Anim. Behav. Process. 25: 92-102.[CrossRef][Medline]
____. 2003. The effect of signaled reinforcement on rats' fixed-interval responding. J. Exp. Anal. Behav. 79:367 -382.[Medline]
Rescorla, R.A. 1990a. Evidence for an association between the discriminative stimulus and the response-outcome association in instrumental learning. J. Exp. Psychol. Anim. Behav. Process. 16:326 -334.[CrossRef][Medline]
____. 1990b. The role of information about the response-outcome relation in instrumental discrimination learning. J. Exp. Psychol. Anim. Behav. Process. 16:262 -270.[CrossRef][Medline]
____. 1994. Control of instrumental performance by Pavlovian and instrumental stimuli. J. Exp. Psychol. Anim. Behav. Process. 20:44 -50.[CrossRef][Medline]
Rescorla, R.A. and Holland, P.C. 1982. Behavioral studies of associative learning in animals. Annu. Rev. Psychol. 33:265 -308.[CrossRef]
Rescorla, R.A. and Solomon, R.L. 1967. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol. Rev. 74:151 -182.[Medline]
Ressler, K.J., Paschall, G., Zhou, X.L., and Davis, M.2002
. Regulation of synaptic plasticity genes during
consolidation of fear conditioning. J. Neurosci.
22:7892
-7902.
Rosen, S.C., Weiss, K.R., and Kupfermann, I. 1979.
Response properties and synaptic connections of mechanoafferent neurons in
cerebral ganglion of Aplysia. J.
Neurophysiol. 42:954
-974.
Rosen, S.C., Teyke, T., Miller, M.W., Weiss, K.R., and Kupfermann, I. 1991. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J. Neurosci. 11:3630 -3655.[Abstract]
Schafe, G.E., Nader, K., Blair, H.T., and LeDoux, J.E.2001 . Memory consolidation of Pavlovian fear conditioning: A cellular and molecular perspective. Trends Neurosci. 24:540 -546.[CrossRef][Medline]
Schwarz, M. and Susswein, A.J. 1986. Identification of the neural pathway for reinforcement of feeding when Aplysia learn that food is inedible. J. Neurosci. 6:1528 -1536.[Abstract]
Skinner, B.F. 1935. Two types of conditioned reflex and a pseudo type. J. Gen. Psychol. 12: 66-77.
____. 1937. Two types of conditioned reflex: A reply to Konorski and Miller. J. Gen. Psychol. 16:272 -279.
____. 1938. The behavior of organisms. Appleton, New York.
Susswein, A.J. and Schwarz, M. 1983. A learned change of response to inedible food in Aplysia. Behav. Neural Biol. 39:1 -6.[Medline]
Susswein, A.J., Gev, S., Feldman, E., and Markovich, S.1983 . Activity patterns and time budgeting of Aplysia fasciata under field and laboratory conditions. Behav. Neural Biol. 39:203 -220.[Medline]
Susswein, A.J., Weiss, K.R., and Kupfermann, I. 1984. Internal stimuli enhance feeding behavior in the mollusc Aplysia. Behav. Neural Biol. 41:90 -95.[Medline]
Susswein, A.J., Schwarz, M., and Feldman, E. 1986. Learned changes of feeding behavior in Aplysia in response to edible and inedible foods. J. Neurosci. 6:1513 -1527.[Abstract]
Thorndike, E.L. 1911. Animal intelligence. Macmillan, New York.
Trapold, M.A. and Overmier, J.B. 1972. The second learning process in instrumental conditioning. In Classical conditioning II: Current research and theory (eds. A.H. Black and W.F. Prokasy), pp. 427-452. Appleton-Century-Crofts, New York.
Trapold, M.A. and Winokur, S. 1967. Transfer from classical conditioning and extinction to acquisition, extinction, and stimulus generalization of a positively reinforced instrumental response. J. Exp. Psychol. 73:517 -525.[Medline]
Trapold, M.A., Lawton, G.W., Dick, R.A., and Gross, D.M.1968 . Transfer of training from differential classical to differential instrumental conditioning. J. Exp. Psychol. 76:568 -573.[Medline]
Walters, E.T. and Byrne, J.H. 1983. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science 219:405 -408.[Medline]
Williams, B.A. 1975. The blocking of reinforcement control. J. Exp. Anal. Behav. 24:215 -225.
____. 1989. Signal duration and suppression of operant responding by free reinforcement. Learn. Motiv. 20:335 -357.
____. 1999. Associative competition in operant conditioning: Blocking the response-reinforcer association. Psych. Bull. Rev. 6:618 -623.
Williams, B.A. and Heyneman, N. 1982. Multiple determinants of blocking effects on operant behavior. Anim. Learn. Behav. 10:72 -76.
Williams, B.A., Preston, R.A., and DeKervor, D.E.1990 . Blocking of the response-reinforcer association additional evidence. Learn. Motiv. 21:379 -398.
Xin, Y., Weiss, K.R., and Kupfermann, I. 1995. Distribution in the central nervous system of Aplysia of afferent fibers arising from cell bodies located in the periphery. J. Comp. Neurol. 359:627 -643.[Medline]