Operant conditioning in invertebrates
Björn Brembs
Learning to anticipate future events on the basis of past experience with the consequences of one's own behavior (operant conditioning) is a simple form of learning that humans share with most other animals, including invertebrates. Three model organisms have recently made significant contributions towards a mechanistic model of operant conditioning, because of their special technical advantages. Research using the fruit fly Drosophila melanogaster implicated the ignorant gene in operant conditioning in the heat-box, research on the sea slug Aplysia californica contributed a cellular mechanism of behavior selection at a convergence point of operant behavior and reward, and research on the pond snail Lymnaea stagnalis elucidated the role of a behavior-initiating neuron in operant conditioning. These insights demonstrate the usefulness of a variety of invertebrate model systems to complement and stimulate research in vertebrates.
Addresses:
Department of Neurobiology and Anatomy, The University of Texas-Houston Medical School, 6431 Fannin, MSB 7.312 Houston, Texas, 77030, USA e-mail: bjoern@brembs.net

Abbreviations
| CPG | central pattern generator |
|---|---|
| RPeD1 | right pedal dorsal 1 |
| RSK | ribosomal S6 kinase |
| US | unconditioned stimuli |
Introduction
We all experience pleasurable and painful events on a daily basis. Predicting the occurrence of either is crucial for seeking the pleasurable ones and avoiding the painful ones. Most of the time, past experience with reliable predictors of these events helps us to do this. We may smell fresh coffee brewing in the morning, hear the sound of a dentist's drill in the waiting room or see dark clouds before a rainstorm. We also experience that touching a hot plate is painful and that saying ‘please' will often give us the desired treat. The learning by which we associate external predictors (conditioned stimuli, CSs) with important outcomes (unconditioned stimuli, USs) is called classical or Pavlovian conditioning [1]. Learning from the consequences of our behavior (an internal predictor) is called operant or instrumental conditioning [2].
Understanding the neurobiology that underlies classical conditioning is a lot easier than doing the same for operant conditioning: one can follow the stimuli from their respective sensory organs into the brain and find the points of convergence where the learning takes place. By contrast, the points where the US (reinforcement or punishment in the operant nomenclature) converges on operant behavior have proven much more elusive. The complexity of the vertebrate brain makes it difficult to discern the circuits that are responsible for the generation of the behavior, and stimuli are processed in several hierarchical and interlocking steps. Fortunately, small brains can also learn operantly and classically. It seems that these simple forms of predictive learning are so fundamental that they appeared early in evolution and have been indispensable ever since. Out of the many invertebrates that show operant conditioning, three in particular have recently helped to further our progress towards a mechanistic model of operant conditioning: the fruit fly Drosophila melanogaster, the sea slug Aplysia californica and the pond snail Lymnaea stagnalis.
Heat-box learning in Drosophila
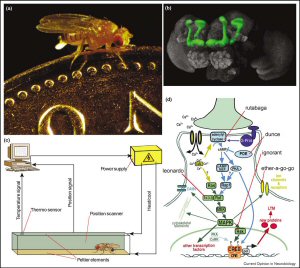
Research on the genetically renowned fruit fly Drosophila (Figure 1a) has been revealing a steady flflow of genes that are involved in olfactory classical conditioning for the past three decades [3–11]. Many of these genes affect the level of the second-messenger cAMP and are preferentially expressed in a prominent neuropil in the flfly's brain, the mushroom bodies (Figure 1b; [12–14]). For some of these genes, being expressed exclusively in the mushroom bodies is sufficient for normal learning [15]. Are those genes also involved in operant conditioning? What role do the mushroom bodies play in operant conditioning?.
The heat-box (Figure 1c) is the perfect instrument to use the powerful genetic techniques in Drosophila to study operant conditioning [16,17,18] . Every time the flfly walks into the designated half of the tiny dark chamber the whole space is heated. As soon as the animal leaves the punished half, the chamber temperature reverts to normal. After a few minutes, the animals restrict their movements to one-half of the chamber, even if the heat is switched off. Several training sessions interspersed by test phases in which the heat is permanently switched off are more effective than one long training session [18]. With a brief reminder training, this memory is still detectable even if the flfly is taken out of the chamber and then tested in a different one, up to two hours later [18].
As it is completely dark in the chamber, the animal is most likely can be shown that the operant memory consists of two to rely on idiothetic cues for orientation, thus minimizing components, a spatial component and a ‘stay-where-you-the contamination with potential classical predictors. It are' component [18].
Flies with mutations in the genes involved in classical conditioning (those affecting cAMP) show marked deficits in the heat-box [16]. However, the question remains of whether learning classical (external) predictors is really the same as learning operant (internal) predictors on the genetic scale or are there operant learning genes that are not involved in classical conditioning? Taking advantage of the size of the fruitflfly, there are usually a barrage of chambers connected in one setup, making a genetic mutant screen possible in an operant learning paradigm for a single flfly. Apparently supplementing the previous mutant data, one of the mutants found using the heat box approach affects an enzyme that is thought to be downstream of the cAMP pathway: the ignorant gene codes for the p90 ribosomal S6 kinase (RSK, Figure 1d; [19]).
However, if the different alleles generated by the screen are scrutinized a little closer, it appears that ignorant has very different effects on operant and classical conditioning. The original mutant (ignP1), with a Drosophila transposable element in the first exon of the gene, shows a sexual dimorphism in the heat-box, where males are impaired but females appear normal [19]. Both males and females of that line are statistically indistinguishable from the wild type controls in olfactory classical conditioning [20]. The null mutant (ign58/1), in which the entire RSK sequence is missing, shows decreased learning and memory in the classical case [20] , but is normal in the heat-box [19]. Finally, several partial deletions of the ignorant gene make flies deficient in the heat-box task [19], but these lines have not yet been tested for classical conditioning. Apparently, different mutations of the ignorant gene have different effects on operant and classical conditioning, which indicates a differentiated role of RSKs in the two forms of learning.
Paralleling the differential results on the molecular scale, there are several operant learning situations, including the heat-box, that do not require the mushroom bodies [21], whereas olfactory classical conditioning is abolished without mushroom bodies [22]. It seems unlikely, however, that the mushroom bodies are generally required for classical conditioning, as flflies without them do very well in classical conditioning with visual CSs [21]. The picture that emerges suggests that the mushroom bodies are needed for chemosensory learning and higher-order integrative tasks [23] . Although the cAMP cascade and its downstream targets are both necessary and sufficient in the mushroom bodies for these tasks [15,22], in operant conditioning they are involved in neurons outside the mushroom bodies and in a different way than in classical conditioning [19].
Neither the neurotransmitter mediating the reinforcement nor the brain region controlling the relevant behaviors are yet known. The antennal lobes, the median bundle, and the ventral ganglion in the thorax are good candidate regions, because a functional cAMP cascade in these regions alone is sufficient for learning in the heat-box [24]. Finding the transmitter and scrutinizing the expression patterns of the wild type and mutated ignorant gene should help to identify the location of the potential regions in which behavior and reinforcement converge. Once those convergence points are found, they can be targeted and specific parts of the molecular machinery can be manipulated to not only evaluate necessity and sufficiency of each point but also to help construct a mechanistic model of operant conditioning on the cellular and molecular level.
Operant reward-learning of feeding behavior in Aplysia
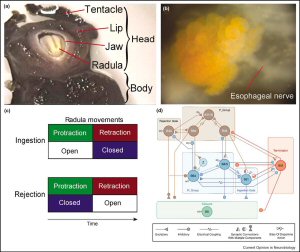
Similar to Drosophila, the sea slug Aplysia (Figure 2a) is also better known for its prominent role in classical conditioning [25–29]. Unlike the situation in Drosophila, its strengths lie in the analysis of the cellular and network level, which provides a possibility to fifind the convergence points of operant behavior and reinforcement. By virtue of its large neurons (Figure 2b) it is possible to trace the neural networks in the ganglia and follow the flflows of activity generated by sensory stimulation or during behavior. Aplysia's feeding behavior (Figure 2c) has proven very valuable for the study of operant conditioning [30, 31–34]. The key neurons in the central pattern generator (CPG) are known [35,36]. They are located in the buccal ganglion and, in part, control the ingestion and rejection movements of the radula (a tongue-like organ) in the buccal mass (Figure 2d). Conveniently, the behavior can be both classically and operantly conditioned [30, 31, 37, 38].
Early on, the esophageal nerve appeared to be crucial for the effectiveness of these conditioning experiments [31,38,39]. Recording extracellularly from the esophageal nerve in the intact animal during a biting movement that fails to grasp food reveals little activity. However, when the animal grasps and swallows seaweed, there are bursts of activity in the esophageal nerve during and outlasting the swallowing movements [30]. Presumably, the esophageal nerve transmits information about the presence of food to the buccal ganglia.
In an effort to mimic the food signal as reward in an operant conditioning experiment, the esophageal nerve was stimulated in vivo (in a pattern resembling the recorded activity) whenever the animals produced a bite (no food present). No other stimuli were contingent with the bites, minimizing the contamination with classical components. Just as if this ‘virtual' food rewarded the animals, they produced more bites in a subsequent test session without stimulation than a control group that had received the same stimulation sequences, but independently of their behavior (yoked control) [30].
Apparently, the reward signal from the esophageal nerve converges on the behavior that is generated by activity in the buccal ganglia. However, the question that remains is in which neurons does this happen? One neuron thought to determine whether a radula movement becomes an ingestion or a rejection, is B51 [32,40]. Interestingly, not only is there evidence that B51 is active during the rewarded behavior but it also receives a dopaminergic input from the esophageal nerve [34], that is, the possibility exists that B51 constitutes a convergence point of operant behavior and dopamine-mediated reward. In line with this hypothesis are findings that B51 shows altered biophysical membrane properties after operant conditioning, making it more excitable [30, 32]. Additional evidence comes from a single cell analogue of operant conditioning. If cultured B51 cells receive an iontophoretic puff of dopamine right after (as opposed to between) depolarization-induced activity that mimics the presumed B51 activity during a bite, they show the same biophysical changes as those seen after operant conditioning [30]. These results are consistent with the view that during this form of operant conditioning, a dopamine-mediated food-reward is contingent on activity in B51 during the rewarded behavior. Activity-dependent plasticity in B51 leads to a modification of the biophysical properties of the neuron that make it more likely to be active. These biophysical changes in B51, in turn, contribute to the increased production of bites seen after operant training.
Although the described biophysical changes are sufficient for some aspects of the operant learning [33], it is not known if the changes in B51 are necessary for learning to occur. It is also not yet known how many more neurons are involved and what their relative contributions are. For example, although B51 is crucial for determining what kind of pattern the buccal CPG produces, it is active rather late during the pattern and not involved in initiating the behavior [32]. To construct a mechanistic model of operant conditioning it will be vital to understand the role of initiating activity and if/how spontaneously active neurons are modified as the learning takes place.
Operant conditioning of aerial respiratory behavior in Lymnaea
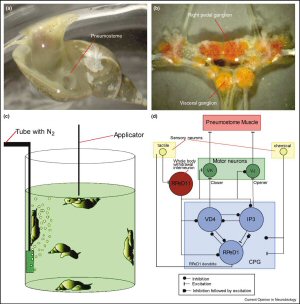
The pond snail, Lymnaea (Figure 3a) may provide the data to elucidate the role of activity initiating neurons in operant conditioning. Lymnaea is a bimodal breather. Under normoxic conditions, it obtains oxygen cutaneously, whereas under hypoxic conditions, it moves to the surface to supplement cutaneous oxygen uptake by aerial respiration using its pneumostome (respiratory orifice; Figure 3a). Similar to Aplysia, Lymnaea has a relatively simple nervous system and a central ring ganglion contains the CPG for generating the aerial respiratory behavior (Figure 2b; [41,42]). Experimentally, hypoxic conditions are induced by bubbling N2 in the training beaker (Figure 3c; [43]). A sharpened wooden applicator is used to lightly touch the pneumostome as it opens. This punishment only causes the animal to close the pneumostome and does not elicit the defensive withdrawal of the whole animal. With repeated stimulation, the animals cease to open their pneumostome. Control groups showed that this effect is neither due to a general decrement caused by the induced hypoxia nor due to non-associative effects of the stimulation [43].
Figure 3 Operant conditioning in Lymnaea. (a) Under hypoxic conditions, the bimodal breather Lymnaea supplements cutaneous respiration with aerial respiration through its pneumostome (image kindly provided by G Spencer). (b) Photograph of the central ring ganglia of Lymnaea (kindly provided by G Spencer). (c) Cartoon of the training apparatus used to operantly condition aerial respiratory behavior in Lymnaea. To create hypoxic conditions, N2 is bubbled through a beaker with pond water for 20 minutes, snails are then added, and training begins after a 10-min acclimatization period. N2 is continuously bubbled throughout the training period. Each time a snail attempts to open its pneumostome it receives a tactile stimulus applied by a sharpened wooden applicator, which causes the pneumostome to close. In the course of the training, the animals learn to suppress the punished respiratory behavior (adapted from [41]). (d) A diagram of the neural circuit (CPG) controlling aerial respiration in Lymnaea. The CPG consists of three identified interneurons. Activity in RPeD1 initiates rhythmogenesis. Activity in interneuron IP3 results in pneumostome opening through monosynaptic excitatory synaptic connections to visceral J (VJ) motor neurons. The monosynaptic excitatory connections to VK motor neurons from interneuron visceral dorsal 4 (VD4) lead to pneumostome closure upon activation of VD4. CPG activity and hence pneumostome muscle activity can be modified by intrinsic (RPeD1 dendrite) and extrinsic (sensory neurons) input to the circuit. Tactile stimulation of the pneumostome area results in the activation of identified mechano-sensory neurons that monosynaptically excite the ‘whole-body withdrawal interneuron', RPeD11 (adapted from [42]). |
The three-cell CPG (Figure 3d) that controls aerial respiratory behavior is well characterized and can be reconstituted in cell culture [44,45]. In the most exten sively characterized invertebrate operant conditioning preparation, various training regimes have been reported to induce a context dependent multi-phasic memory, which includes aspects of short-, intermediate-, and long-term memory, that lasts for up to one month [46, 47, 48, 49, 50–54]. The accounts of the underlying neurobiology include the differential requirements of local translation and transcription for intermediate-and long-term memory, respectively, as well as neural correlates in neurons in the CPG [46, 49, 55, 56]. Importantly, the CPG activity initiating neuron RPeD1 (right pedal dorsal 1; see Figure 3d) shows a lower spontaneous fifiring frequency in semi-intact preparations from trained animals after a brief reminder training [56]. In isolated ganglia of operantly trained animals, RPeD1 is quiescent more often than in preparations from yoked control animals, and the efificacy of the excitatory connection from RPeD1 to IP3 (input 3 interneuron; see Figure 3d) is reduced [55].
The most parsimonious explanation of the published data is that RPeD1 is active at the beginning of the behavior and contingent stimulation of the pneumostome changes several of its biophysical and synaptic properties. These changes can last up to several hours solely relying on local translation. Transcription is required in order for the changes to become more permanent. It would be very interesting to see if these changes could be brought about in a single cell analog with the isolated RPeD1 in cell culture. For such an experiment, it remains to be determined of what biophysical or molecular nature the changes found in RPeD1 are, and whether the punishment is mediated through the whole-body withdrawal neuron (Figure 3d) or affects RPeD1 directly.
Conclusions
Using the particular advantages of each model system, research in Drosophila generated new insights into the molecular processes involved in operant conditioning, research in Aplysia yielded a convergence point of operant behavior and reinforcement and suggested a possible cellular mechanism of operant conditioning, and research in Lymnaea shed some light on the possible role of activity-initiating neurons in a CPG in operant conditioning. It is noteworthy that each animal provided a piece of data that was not obtainable in the other model systems. If one were to attempt an integrated mechanistic model of operant conditioning at this early stage, one could say that contingent reinforcement/punishment acts on behavior-initiating and -switching neurons altering both their biophysical membrane properties and their synaptic connections through the cAMP cascade and its downstream targets.
Paralleling evidence from vertebrates [57,58], it was found that different brain circuits and molecular mechanisms are involved when external (i.e. stimuli) or internal (i.e. behaviors) predictors are used to anticipate important events. Not unexpectedly, the data presented here are consistent with the idea that the modifications induced by learning reside in the circuits involved in processing the predictors, that is, the sensory pathways in classical conditioning and the behavior generators (CPGs) in operant conditioning. On a more general level, one can conceive that any important event (US) generates a distributed signal acting on coincidentally active neurons through activity-dependent plasticity. The location of the circuits, the points of convergence between the neurons processing the events that precede the signal (the predictors) and the signal itself will depend not only on the type of predictor (operant or classical) but also on the type of US (reward or punishment).
Research in vertebrates is not so fortunate to be able to deduce the involvement of the relevant brain regions by virtue of their location with respect to the studied learning paradigm. Most progress towards a mechanistic model of operant conditioning can be made by studying several model organisms, so questions can be tackled on many levels of complexity with the ideal method in the ideal system for the particular question. Thus, the largest leaps in understanding operant conditioning can be expected from integrative approaches using the common task of learning from the consequences of behavioral actions to constrain the design of experiments, so that they become comparable across phyla. Using the common and disparate data, one can then construct a general mechanistic model of operant conditioning, with wide ramifications ranging from the basic sciences of neurobiology and evolution to substance abuse, mental illness and even philosophy.
Acknowledgements
I am indebted to R Mozzachiodi, E Antzoulatos, G Phares, F Lorenzetti, D Baxter, G Spencer and G Putz for commenting on an earlier version of the manuscript, to J Byrne and D Baxter for providing laboratory space and discussions, to G Spencer, J Dow, M Heisenberg and D Baxter for providing fifigures, and to the Emmy-Noether program of the Deutsche Forschungsgemeinschaft for fifinancial support.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- Pavlov IP: Conditioned Reflexes. Oxford: Oxford University Press; 1927.
- Thorndike EL: Animal Intelligence. New York: Macmillan; 1911.
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S: Dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA 1976, 73:1684-1688.
- Quinn WG, Sziber PP, Booker R: The Drosophila memory mutant amnesiac. Nature 1979, 277:212-214.
- Byers D, Davis RL, Kiger JA Jr: Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature 1981, 289:79-81.
- Duerr JS, Quinn WG: Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc Natl Acad Sci USA 1982, 79:3646-3650.
- Tully T, Quinn WG: Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985, 157:263-277.
- Davis RL, Dauwalder B: The Drosophila dunce locus: learning and memory genes in the flfly. Trends Genet 1991, 7:224-229.
- Davis RL, Cherry J, Dauwalder B, Han PL, Skoulakis E: The cyclic AMP system and Drosophila learning. Mol Cell Biochem 1995 :271-278.
- Feany MB, Quinn WG: A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science 1995, 268:869-873.
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG: The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 2000, 103:805-813.
- Nighorn A, Healy MJ, Davis RL: The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron 1991, 6:455-467.
- Han PL, Levin LR, Reed RR, Davis RL: Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron 1992, 9:619-627.
- Skoulakis EM, Kalderon D, Davis RL: Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron 1993, 11:197-208.
- Zars T, Fischer M, Schulz R, Heisenberg M: Localization of a short-term memory in Drosophila. Science 2000, 288:672-675.
- Wustmann G, Rein K, Wolf R, Heisenberg M: A new paradigm for operant conditioning of Drosophila melanogaster. J Comp Physiol [A] 1996, 179:429-436.
- Wustmann G, Heisenberg M: Behavioral manipulation of retrieval in a spatial memory task for Drosophila melanogaster. Learn Mem 1997, 4:328-336.
- ** Putz G, Heisenberg M: Memories in Drosophila heat-box learning. Learn Mem 2002, 9:349-359.
The authors dissect the Drosophila heat-box learning into two components. They also demonstrate the associative nature of heat-box learning with transfer tests and reveal a two hour memory that is independent of the mushroom bodies. - * Putz G: Characterization of memories and ignorant (S6KII) mutants in operant conditioning in the heat-box [PhD thesis].� Wu¨ rzburg: Julius-Maximilians-Universität Würzburg; 2003. [URL: http://opus.bibliothek.uni-wuerzburg.de/opus/volltexte/2003/419/pdf/Thesisgesamt.pdf]
A detailed account of the specifics of the Drosophila P-Element mutant screen for operant learning mutants in the heat-box. The author characterizes the various mutants of the ignorant gene genetically and behaviorally. The study emphasizes the molecular genetic tools in the study of operant conditioning. - Bertolucci F: Characterization of learning and memory in Drosophila S6Kii (ignorant) mutants in classical conditioning [Tesi di laurea (Bachelor thesis)]. Padua: Universita´ di Padova; 2003.
- Wolf R, Wittig T, Liu L, Wustmann G, Eyding D, Heisenberg M: Drosophila mushroom bodies are dispensable for visual, tactile and motor learning. Learn Mem 1998, 5:166-178.
- de-Belle JS, Heisenberg M: Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 1994, 263:692-695.
- Heisenberg M: Mushroom body memoir: from maps to models. � Nat Rev Neurosci 2003, 4:266-275. The author presents a great review covering the most recent advances in mushroom body research and proposes a general model of mushroom body function.
- Zars T, Wolf R, Davis R, Heisenberg M: Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learn Mem 2000, 7:18-31.
- Buonomano DV, Byrne JH: Long-term synaptic changes produced by a cellular analog of classical conditioning in Aplysia. Science 1990, 249:420-423.
- Carew TJ, Walters ET, Kandel ER: Associative learning in Aplysia: cellular correlates supporting a conditioned fear hypothesis. Science 1981, 211:501-504.
- Kandel ER, Schwartz JH: Molecular biology of learning: modulation of transmitter release. Science 1982, 218:433-443.
- Hawkins RD, Abrams TW, Carew TJ, Kandel ER: A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science 1983, 219:400-405.
- Walters ET, Carew TJ, Kandel ER: Classical conditioning in Aplysia californica. Proc Natl Acad Sci USA 1979, 76:6675-6679.
- ** Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, Byrne JH: Operant reward learning in Aplysia: neuronal correlates and � mechanisms. Science 2002, 296:1706-1709.
This is an Aplysia study that is presented on three levels of complexity: behavior, network and single cell. The authors propose a cellular mechanism in an identified neuron in which operant behavior and reward converge. The study emphasizes the selection of rewarded behaviors. - Nargeot R, Baxter DA, Byrne JH: Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J Neurosci 1997, 17:8093-8105.
- Nargeot R, Baxter DA, Byrne JH: In vitro analog of operant conditioning in Aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron. J Neurosci 1999, 19:2247-2260.
- Nargeot R, Baxter DA, Byrne JH: In vitro analog of operant conditioning in Aplysia. II. Modifications of the functional dynamics of an identified neuron contribute to motor pattern selection. J Neurosci 1999, 19:2261-2272.
- Nargeot R, Baxter DA, Patterson GW, Byrne JH: Dopaminergic synapses mediate neuronal changes in an analogue of operant conditioning. J Neurophysiol 1999, 81:1983-1987.
- Elliott CJ, Susswein AJ: Comparative neuroethology of feeding control in molluscs. JExp Biol 2002, 205:877-896.
- Cropper EC, Weiss KR: Synaptic mechanisms in invertebrate pattern generation. Curr Opin Neurobiol 1996, 6:833-841.
- Lechner HA, Baxter DA, Byrne JH: Classical conditioning of feeding in Aplysia: II. Neurophysiological correlates. J Neurosci 2000, 20:3377-3386.
- Lechner HA, Baxter DA, Byrne JH: Classical conditioning of feeding in Aplysia: I. Behavioral analysis. J Neurosci 2000, 20:3369-3376.
- Schwarz M, Susswein AJ: Identification of the neural pathway for reinforcement of feeding when Aplysia learn that food is inedible. J Neurosci 1986, 6:1528-1536.
- Plummer MR, Kirk MD: Premotor neurons B51 and B52 in the buccal ganglia of Aplysia californica: synaptic connections, effects on ongoing motor rhythms, and peptide modulation. J Neurophysiol 1990, 63:539-558.
- Lukowiak K, Sangha S, McComb C, Varshney N, Rosenegger D, Sadamoto H, Scheibenstock A: Associative learning and memory in Lymnaea stagnalis: how well do they remember? J Exp Biol 2003, 206:2097-2103.
- Taylor BE, Lukowiak K: The respiratory central pattern generator of Lymnaea: a model, measured and malleable. Respir Physiol 2000, 122:197-207.
- Lukowiak K, Ringseis E, Spencer G, Wildering W, Syed N: Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalis. J Exp Biol 1996, 199:683-691.
- Syed NI, Bulloch AG, Lukowiak K: In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science 1990, 250:282-285.
- Lukowiak K: Experimental reconstruction of neuronal pattern generators. Curr Opin Neurobiol 1991, 1:577-582.
- *Sangha S, Scheibenstock A, McComb C, Lukowiak K: Intermediate and long-term memories of associative � learning are differentially affected by transcription versus translation blockers in Lymnaea. J Exp Biol 2003, 206:1605-1613.
Confirming and detailing the results from Scheibenstock et al. [49] ,the authors report that intermediate-term memory in the Lymnaea preparation depends on local mRNA translation, whereas long-term memory depends on transcription. - Sangha S, McComb C, Lukowiak K: Forgetting and the extension of memory in Lymnaea. J Exp Biol 2003, 206:71-77.
- Smyth K, Sangha S, Lukowiak K: Gone but not forgotten: the lingering effects of intermediate-term memory on the persistence of long-term memory. J Exp Biol 2002, 205:131-140.
- * Scheibenstock A, Krygier D, Haque Z, Syed N, Lukowiak K: The soma of RPeD1 must be present for long-term memory � formation of associative learning in Lymnaea. J Neurophysiol 2002, 88:1584-1591.
The authors report that ablating the soma of RPeD1 prevents long-term memory formation in Lymnaea, but leaves already established memory intact. - Sangha S, McComb C, Scheibenstock A, Johannes C, Lukowiak K: The effects of continuous versus partial reinforcement schedules on associative learning, memory and extinction in Lymnaea stagnalis. J Exp Biol 2002, 205:1171-1178.
- McComb C, Sangha S, Qadry S, Yue J, Scheibenstock A, Lukowiak K: Context extinction and associative learning in Lymnaea. Neurobiol Learn Mem 2002, 78:23-34.
- Haney J, Lukowiak K: Context learning and the effect of context on memory retrieval in Lymnaea. Learn Mem 2001, 8:35-43.
- Lukowiak K, Adatia N, Krygier D, Syed N: Operant conditioning in Lymnaea: evidence for intermediate-and long-term memory. Learn Mem 2000, 7:140-150.
- Lukowiak K, Cotter R, Westly J, Ringseis E, Spencer G: Long-term memory of an operantly conditioned respiratory behaviour pattern in Lymnaea stagnalis. J Exp Biol 1998, 201:877-882.
- Spencer GE, Syed NI, Lukowiak K: Neural changes after operant conditioning of the aerial respiratory behavior in Lymnaea stagnalis. J Neurosci 1999, 19:1836-1843.
- * Spencer GE, Kazmi MH, Syed NI, Lukowiak K: Changes in the activity of a CPG neuron after the reinforcement of an operantly � conditioned behavior in Lymnaea. J Neurophysiol 2002, 88:1915-1923.
The authors find reduced firing frequency in the activity-initiating neuron RPeD1 after operant conditioning of aerial respiratory behavior in Lymnaea. - Corbit LH, Balleine BW: Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process 2003, 29:99-106.
- Corbit LH, Muir JL, Balleine BW: The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci 2001, 21:3251-3260.
- Impey S, Obrietan K, Storm DR: Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 1999, 23:11-14.