|
Drosophila
as a new model organism for the Summary: We report here the effects of several neurobiological determinants on aggressive behaviour in the fruitfly Drosophila melanogaster. This study combines behavioural, transgenic, genetic and pharmacological techniques that are well established in the fruitfly, in the novel context of the neurobiology of aggression. We find that octopamine, dopamine and a region in the Drosophila brain called the mushroom bodies, have profound influence on the expression of aggressive behaviour. Serotonin had no effect. We conclude that Drosophila, with its advanced set of molecular tools and its behavioural richness, has the potential to develop into a new model organism for the study of the neurobiology of aggression. Introduction Drosophila is the “jack of all trades” in biology, but has not been studied in the context of the neurobiology of aggression. The fruitfly exhibits aggressive behaviour (Jacobs, 1960) and this behaviour is ethologically well characterized (Dow and von Schilcher, 1975; Jacobs, 1978; Lee and Hall, 2000; Skrzipek et al., 1979). The evolutionary relevance of this aggressive behaviour is also well established (Boake and Hoikkala, 1995; Boake and Konigsberg, 1998; Boake et al., 1998; Dow and von Schilcher, 1975; Hoffmann, 1988, 1989, 1994; Hoffmann and Cacoyianni, 1989; Ringo et al., 1983; Skrzipek et al., 1979; Zamudio et al., 1995). Finally, the ecological circumstances under which Drosophila exhibits territoriality and aggression have been examined in great detail (Hoffmann, 1987, 1988, 1989, 1994; Hoffmann and Cacoyianni, 1989, 1990). Under appropriate conditions, male flies try to occupy a food patch and defend it against other males, even in the laboratory. However, this aggressive behaviour in Drosophila has escaped most neurobiologists’ notice. In this article, we combine ethological, ecological and evolutionary knowledge with molecular, genetic and pharmacological tools to manipulate the aggressive behaviour of Drosophila melanogaster. To our knowledge, only two genetic factors have been reported to affect aggressive behaviour in Drosophila: The sex-determination hierarchy (SDH) and the beta-alanine pathway. fruitless (fru) and dissatisfaction (dsf) mutants have been described as more aggressive than wildtype controls (Lee and Hall, 2000). Both genes are part of the SDH. Flies carrying mutant alleles of the black (b) gene appear less aggressive, whereas ebony (e) mutants appear more aggressive (Jacobs, 1978). The enzymes encoded by the two genes regulate beta-alanine levels (b flies have reduced, e flies elevated levels).
It is straightforward to expect genes of the SDH to affect sex-specific behaviours, but the pathways through which they modulate that behaviour are largely unknown. One possibility could be via the regulation of small neuroactive molecules (such as beta-alanine and the biogenic amines) and their receptors. Biogenic amines play a key role in the regulation of aggressive behaviour not only in vertebrates, but also in arthropods (e.g. Edwards and Kravitz, 1997; Heinrich et al., 1999, 2000; Huber et al., 1997a, b; Kravitz, 2000; Schneider et al., 1996; Stevenson et al., 2000). The biogenic amine system in flies is well described (see Monastirioti, 1999). Most serotonin and dopamine mutants in Drosophila are either lethal or affect both serotonin and dopamine, due to their shared synthesis pathways (e.g. Johnson and Hirsh, 1990; Lundell and Hirsh, 1994; Shen et al., 1993; Shen and Hirsh, 1994). However, there are established protocols that are commonly used to manipulate the levels of these amines individually in the adult fly (Neckameyer, 1998; Vaysse et al., 1988). Octopamine null mutants have been generated and characterized (Monastirioti et al., 1996). Interestingly, certain octopamine and dopamine receptors are preferentially expressed in a prominent neuropil in the Drosophila brain called the mushroom bodies (Han et al., 1996, 1998). Thus, all of the prerequisites for a systematic analysis of the neurobiological factors involved in the expression of aggressive behaviour are available: 1) a considerable body of knowledge about the behaviour and its ecological context, 2) circumstantial evidence about possible neurobiological factors involved in regulating the behaviour, and 3) methods for manipulating these factors and for quantifying the behaviour. In a first attempt to characterize the effects of various candidates for neurobiological factors regulating aggression, we report here the results of a competition experiment. Six male flies competed for a food patch and three mated females. The experimental males have been manipulated either by a classical mutation affecting beta-alanine levels, a P-element mutation affecting octopamine levels, insertion of transgenes affecting synaptic output from the mushroom bodies or by pharmacological treatment affecting serotonin or dopamine levels and then tested for their aggressive behaviour. Materials and Methods Flies: Animals were kept on standard cornmeal/molasses
medium (see Guo et al., 1996 for recipe) at 25° C and 60% humidity
with a 16hr light/8hr dark regime, except where noted. The females
in all experiments were mated wildtype Canton S flies.
Mutants: Black1 and ebony1 mutant strains from the laboratory’s 18° C stock collection (provided by S. Benzer in 1970) were kept at 25° C for at least two generations. The M18 P-element octopamine mutant and control stocks (Monastirioti et al., 1996) were kept at 25° C for two generations after arrival. Transgenes: Sweeney et al. (1995) developed a method that constitutively blocks synaptic transmission by expressing the catalytic subunit of bacterial tetanus toxin (Cnt-E) in target neurons in the Drosophila brain using the P[GAL4] technique (Brand and Perrimon, 1993). Inspired by the preferential expression of certain dopamine and octopamine receptors in the mushroom bodies (Han et al., 1996, 1998), we used the Cnt-E transgene to block synaptic output from the mushroom bodies (Sweeney et al., 1995). Expression of another transgene, an inactive form of the tetanus toxin light chain (imp-tntQ), controlled for deleterious effects of protein overexpression (Sweeney et al., 1995). The P[GAL4] line mb247 (Schulz et al., 1996) served as a mushroom body specific GAL4 driver (Zars et al., 2000) for both toxins. The trans-heterozygote offspring from the GAL4 driver strain and the two UASGAL4 reporter strains (Cnt-E and imp-tntQ) entered the study. Pharmacological treatments: Drosophila from the wildtype strain Berlin (wtb) were treated as described by Neckameyer (1998) and Vaysse et al. (1988). Briefly, the animals were fed a sucrose solution containing either 10mg/ml of the serotonin precursor 5HTP (5-hydroxy-tryptophan), or 10mg/ml of the serotonin synthesis inhibitor pCPA (para-chlorophenylalanine) to manipulate serotonin levels. Effectiveness of the treatment was verified qualitatively with standard immunohistochemical techniques using rabbit serotonin antisera (see inset; Buchner et al., 1986, 1988). Alternatively, the animals were treated with 1mg/ml of the dopamine precursor L-DOPA (L-3,4-dihydroxyphenylalanine) or 10mg/ml of the dopamine synthesis inhibitor 3IY (3-iodo-tyrosine) to manipulate dopamine levels. Effectiveness of the treatment was verified by observation of cuticle tanning. A dose of 10mg/ml L-DOPA was lethal, confirming unpublished data from Wendy Neckameyer (Saint Louis University School of Medicine). Experimental groups: Using the different stocks described above, we arranged six different groups of ‘low’ vs. ‘high’ males, such that the respective amine or the amount of synaptic output from the mushroom bodies was manipulated to produce relative high and low level subgroups.
Thus, we arranged four experimental groups and two control groups. For each group, the two subgroups ('high' and 'low') compete against each other in one recording chamber. Each group was tested twice with different animals. Recording chambers: Aggression was studied in cylindrical cages similar to those used by Hoffmann (1987): 100mm Petri-Dishes, top and bottom separated by a 40mm high spacer (i.e. a cylindrical chamber of 100mm diameter and 40mm height). The bottom of the chamber was filled with 2% Agar to moisturize the chamber. Flies were introduced by gentle aspiration through a small hole in the spacer. A food patch (10mm diameter, 12mm high) was positioned in the centre of the chamber, containing a mixture of minced 2% agar, apple juice, syrup and a live yeast suspension (after Reif, 1998), filled to level with the rim of the containing vial. The chamber was placed in a Styrofoam box (used to ship biochemical reagents on dry-ice; outer measures: 275x275mm, height: 250mm, inner measures: 215x215mm, height: 125mm) to standardize lighting conditions and to shield the chambers from movements by the experimenters. Two Styrofoam boxes with one chamber each were arranged underneath video cameras, focused on the food patch in a darkened room at 25°C. Ring-shaped neon-lights (Osram L32W21C, power supply Philips BRC406) on top of the boxes provided homogenous illumination throughout the experiment.
Experimental time course: The stocks were treated completely in parallel (see Table 1). A 5% sucrose solution (in Drosophila ringer) with or without added treatment was pipetted onto 5 pieces of filter paper snugly fitting in cylindrical (12x40mm) vials before transferring newly ecclosed (0-24 hours) male flies into the vials. The flies were transferred into new vials with new solution and new filter paper on a daily basis for 5 days. Each group was treated in two replicates, starting with new flies on different days (see Table 1). On the 5th day, 4-6 flies per subgroup were briefly immobilised on a cold plate and marked on the thorax with either green or white acrylic paint (one small dot). At 08.00hrs (1 hour after lights-on) on the 6th day, the animals of the two groups treated in parallel were transferred into the recording chambers (3 mated but otherwise untreated Canton S females and 6 males; 3 males of each paired subgroup) and placed underneath the video cameras with all conditions identical to those during the recording time, except the VCRs were turned off. Continuing the parallel treatment of two groups per day, two video set-ups were used simultaneously (‘left’ and ‘right’). After an acclimatisation period of 2 hours, the VCRs were set to record. For each group, we recorded four hours of fly behaviour, once in each location (yielding the two replicates for each group), resulting in 12 video tapes (see Table 2). Data from both replicates were pooled. Since each group was measured twice with 6 (3+3) experimental animals (males) for each recording, the total number of observed males was 6 animals x 2 replicates x 6 groups = 72. Recording of the experiments was randomised across days.
Behavioural scoring: Only male-male interactions were counted.
Mated females lose their receptivity to male advances and the males
cease courting quickly, refraining from courting for a number of hours
(courtship conditioning; e.g. Greenspan and Ferveur, 2000). Little
courtship behaviour was thus observed after the acclimatisation period. 1: high attacks high
aggressive encounter (1aggr) This design thus yielded 7 values, one value for each of the respective interaction categories, giving each of the 6 groups a characteristic aggression-profile (Fig. 1A). Data analysis: A log-linear analysis (delta=0.005, criterion for convergence=0.0005, max. iterations 500) was performed over the 6x7 table of observed behavioural frequencies to determine the effect of the treatments on the distribution of behavioural classes. To normalize for the total number of encounters, two derived parameters were computed from the raw data. The first is the likelihood that an individual of one subgroup will attack during an encounter (attack probability, P[A]). It is calculated as the fraction of all encounters in that group involving a 'high' (or 'low', respectively) animal, where such an animal was the aggressor:
Thus, P[A] describes the probability that a given individual
will act aggressively against any other individual it encounters. The
second derived parameter assesses the representation of each subgroup
in the total number of encounters (encounter probability, P[E]). It
is calculated analogously to the first parameter as the fraction of
all encounters in a group, where an animal of a specific subgroup (i.e.
‘high’ or ‘low’) participated:
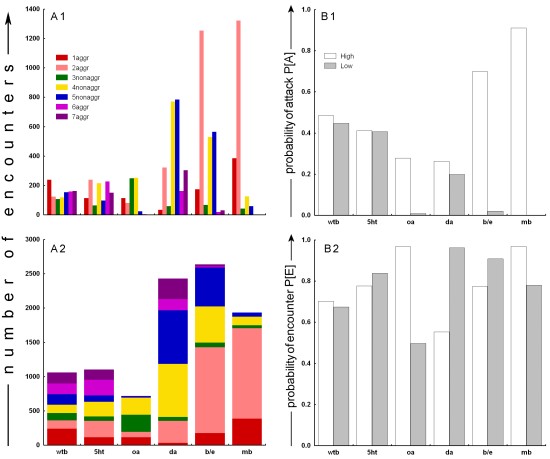
Thus, P[E] describes the probability that an individual of one subgroup will be a participant in an encounter. While the P[A] can be said to describe the level of aggression of a certain subgroup, the P[E] can be perceived as a control measure for the overall number of interactions in that subgroup, as influenced by e.g. general activity, visual acuity etc. After the data transformation, the resulting probabilities were tested against random distribution using c2 tests. Results We performed two 4 hour experiments with 4 experimental and 2 control groups in each experiment. In all, 48 hours of video tape were analysed containing 9881 encounters (an average of 3.4 enc./min or 137.2 enc./male). The two 4 hour experiments were pooled for each group, yielding one 7-score aggression profile for each group (Fig. 1A). A log-linear analysis over the 6 groups and the 7 behavioural classes yields a p<0.0001 (Pearson c²=6479.426, d.f.=30), suggesting the various treatments were effective in changing the proportions of the different classes of encounters in each group. Fig. 1:
Raw and derived data from all six groups. A: Raw behavioural
scores. Two different graphs are depicting the same data in order to
facilitate the interpretation of the complex data structure obtained
from our experiments. A1: multiple bars graph. A2: single
bar graph. See Methods for details on behavioural classification.
B: Derived probabilities. B1: The probability of attacking.
For each subgroup (high, low) the fraction of encounters
where a member of that subgroup was the aggressor is calculated from
the total number of subgroup encounters. B2: The probability
of an encounter. For each subgroup (high, low) the fraction
of encounters (irrespective of classification) in which a member of
that subgroup participated is calculated from the total number of encounters. The raw data (Fig. 1A), reveal that the 2 control groups behaved according to our expectations. The wtb negative control shows a uniform distribution of aggressive encounters, whereas the beta-alanine positive control is skewed towards the mutants with high levels of beta-alanine (Fig. 1A1). The clearest effects among experimental groups were obtained from the octopamine mutants and the mb group. Both octopamine null mutants (oa-) and animals with inhibited mushroom bodies (mb-) are virtually non-aggressive (Fig. 1A). In the representation in Fig 1A2, the octopamine group seems similar to the wildtype control except for the missing values for 6aggr and 7aggr. However, while the oa+ animals appear to show a wildtype level of aggression, the mb+ animals show elevated levels of aggression compared to all other groups (Fig. 1A). It also appears that our serotonin treatment had little effect on aggression (Fig. 1A). The dopamine treatment appears to be somewhat effective in decreasing
the number of aggressive encounters in animals with high levels of dopamine,
while the animals with low levels of dopamine seem to have similar,
if not slightly higher numbers of aggressive encounters than the wildtype
controls. Obviously, the number of non-aggressive encounters in the
dopamine treated animals is strongly elevated (Fig. 1A). Interestingly,
the two subgroups show inverted profiles for intra- and inter-subgroups
aggression (i.e. 1aggr/2aggr and 6aggr/7aggr).
With significant effects of our treatments on the distribution of the behaviours within each group, we can process the data in order to determine the effect of our treatments on the propensity of the animals to become aggressive. The fraction of all encounters involving a ‘subgroup’ animal, where such an animal was the aggressor is calculated (Fig. 1B1; P[A] see Materials and Methods). The P[A] allows us to estimate the effects of the treatments on aggression. On P[A] values, Chi² tests can be computed to test the null hypothesis that our treatments had no effect on the probability of being aggressive. Table 3 summarizes the Chi² results for all six groups. The statistics confirm the effects already visible in the raw data (Fig 1A): the two control groups (wtb and b/e) were consistent with our expectations. The obvious effect of octopamine null mutants being completely non-aggressive is corroborated by our statistical analysis, as are the extreme effects of expressing active and inactive, respectively, tetanus toxin in the flies' mushroom bodies (Fig. 1B1). The serotonin treatment had no significant effect on the probability of the flies becoming aggressive during an encounter, despite the fact that we could verify the effectiveness of the treatment immunohistochemically (see inset in Material and Methods). The group in which the dopamine levels were manipulated shows a moderate, but statistically reliable, effect of high dopamine levels leading to a higher probability to attack in an encounter. Despite most of our treatments having a record of influencing aggression in other animals, the possibility exists that the different treatments may have altered the number of aggressive encounters indirectly by altering the total number of encounters via other factors such as general activity or visual acuity, etc. A likely candidate variable where such effects could be detected is the distribution of encounters over the subgroups, P[E]. For instance, if the treatment rendered the animals of one subgroup inactive, the P[E] of this subgroup should be smaller than that of the other subgroup. If the obtained aggression scores were but a reflection of asymmetric P[E]s, they should follow the pattern of P[E] asymmetry. Fig. 1B2 depicts the distribution of encounters over the two subgroups, independently of encounter classification. Again, c² statistics are performed and summarized in Table 2. All treatments led to a significant asymmetry in P[E] between subgroups, with the exception of the negative wtb controls. However, the pattern of asymmetry does not seem to match the pattern of asymmetry in the level of aggression (see Discussion). Discussion Most importantly for this first study of the effects of various treatments on aggression in Drosophila, the animals in the control groups behaved exactly as expected: no differences were detected among the subgroups of the wtb negative control and previously published higher aggression levels in the ebony (high beta-alanine) than in the black (low beta-alanine) flies (Jacobs, 1978) could be reproduced. These findings corroborate our pilot studies in which we repeatedly observed the same pattern (unpubl. data). Octopamine null mutants exhibit strongly reduced aggression, as do flies with low levels of synaptic output from their mushroom bodies. Interestingly, certain types of octopamine and dopamine receptors are preferentially expressed in the mushroom bodies of wildtype flies (Han et al., 1996, 1998). It is tempting to interpret this phenocopy of the octopamine mutants as resulting from Kenyon cells being the major regulators of octopamine- (and/or dopamine-) mediated aggression. Recently, temperature sensitive shibirets1 constructs have been developed to conditionally block synaptic transmission (e.g. Dubnau et al., 2001; Kitamoto, 2001; McGuire et al., 2001; Waddell et al., 2000). Unfortunately, at the time of our experiments, the shibirets1 constructs were not yet available. Future experiments definitely should include shibirets1 constructs to replicate our mb- results, examine the high levels of aggression in the mb+ flies and look for other brain areas involved in aggression. Replication of our results using the shibirets1 constructs would also eliminate the possible explanation that the expression of tetanus-toxin anywhere in the fly’s brain abolishes aggressive behaviour and solve the problem of UAS promoter leakiness. The octopamine result is conspicuous in another respect: it is consistent with studies in crickets, where depletion of octopamine and dopamine decreases aggressiveness (Stevenson et al., 2000), but it contrasts with studies in crustaceans where high octopamine levels tend to bias behaviour towards submissiveness (Antonsen and Paul, 1997; Heinrich et al., 2000; Huber et al., 1997a). The high aggression observed in the mb+ animals is difficult to interpret. In principle, the inactive toxin should not have any effect on the secretion of neurotransmitter at the synapse. More likely is an insertion effect of the P-element containing the imp-tntQ transgene. In that case it would be extremely interesting to characterize the genetic environment within which the P-element lies in order to find the gene responsible for such aggressiveness. One may argue that high aggressiveness by flies of one subgroup may produce low aggression in the respective other subgroup. In the case of the mb group, this is unlikely, because there still should be at least some aggression between mb- animals, even if mb+ animals attacked every other male they encountered. Moreover, mb- animals seemed unaffected by the repeated attacks from mb+ males and kept coming back to the patch soon after an mb+ male chased it off the patch (this is the reason for the high 2aggr value in Fig. 1). However, mb- animals were never observed to be the aggressor. It thus seems more likely that the high frequency of attacks by mb+ males is due to a combination of high levels of aggression due to insertion effects of the imp-tntQ transgene and returning mb- males repeatedly eliciting aggressive behaviours in the mb+ males. Our serotonin treatment has no significant effect on aggression, despite the fact that we could verify the effectiveness of the treatment immunohistochemically (see inset in Material and Methods). Also, Vaysse et al. (1988) observed effects on learning and memory after identical treatment, indicating that this pharmacological manipulation of serotonin levels in principle can have behavioural effects. Moreover, we observed a noticeable increase in activity in the 5ht- flies, a subjective impression that is corroborated by the significantly increased P[E] of this subgroup (Fig. 1B2). Nevertheless, the possibility remains that the observed difference in serotonin immunoreactivity was not high enough to generate significant differences in aggression though high enough to affect other behaviours. The lack of serotonergic effect on aggression was also repeatedly observed in our pilot studies (unpubl. data). Lee and Hall (2001) have reported that the pattern of serotonergic cells in the Drosophila brain is unaltered in the more aggressive fru mutants, confirming the idea that serotonin is not crucial for regulating aggressive behaviour in the fly. The serotonin results presented here are also consistent with data in crickets, where serotonin depletion appears to have no effect (Stevenson et al., 2000); they contrast with data in crustaceans, where injections of serotonin increase the level of aggressive behaviour (Edwards and Kravitz, 1997; Huber et al., 1997a, b; Kravitz, 2000). Our serotonin data thus parallel our octopamine data in conforming with insect data but contrasting with observations in crustaceans. Perhaps aminergic control of aggression functions fundamentally differently in those two arthropod groups? Our dopamine treatment had complex effects. The absolute number of non-aggressive encounters appears elevated compared to the wildtype controls (Fig. 1A), reducing overall aggression probabilities (Fig. 1B1; P[A]). Also, while the raw data indicate higher aggression scores in the animals with low dopamine (Fig. 1A1), the P[A] is higher in animals with high dopamine levels (Fig. 1B1). Taking the number of encounters that each subgroup experiences (Fig. 1B2, P[E]) into account, it seems as if the higher raw scores for the ‘low’ dopamine animals is generated by the higher P[E] in this subgroup. Once that factor is accounted for (Fig. 1B1), the perceived difference between raw and derived data disappears. A general point of concern is side effects of our treatments. Both e and b flies exhibit varying degrees of visual impairment (unpubl. data and Heisenberg, 1971, 1972; Hovemann et al., 1998; Jacobs, 1978), with e showing more severe defects than b flies (unpubl. data and Jacobs, 1978). Without screening pigments (i.e. white-), the M18 octopamine jump-out mutants are expected to have severely impaired vision compared with the control strain still carrying the P-element. Also, the extent to which the treatments may affect general activity is largely unknown (but see Martin et al., 1998). One may assume that a subgroup’s P[E] should reflect overall activity. Not surprisingly, the more visually impaired e and oa- flies have lower P[E]s than the b and oa+ subgroups, respectively (Fig. 1B2). However, the probability to attack seems entirely unaffected by this measure of general activity, as the relations are reversed. Moreover, both the dopamine and the mushroom body groups show a higher probability to attack in the respective ‘high’ subgroup (Fig. 1B1), but their P[E]s are inverted with respect to their P[A]s (Fig. 1B2). Thus, while both vision and general activity may influence aggression, those factors seem to have only marginal effects compared to the determinants studied here. Of course, this study is only a beginning. We did not address encounter duration, behavioural composition or opponent identity/recognition, let alone potential mechanisms as to how the identified factors might exert their effects. However, one can conclude that our method reproduced published data (the e/b group) and yielded new insights into the neurobiological determinants of aggression in Drosophila melanogaster. Serotonin appears to have no effect, while dopamine, octopamine and the mushroom bodies could be linked to the promotion of aggressive behaviour. We hope to inspire others to exploit Drosophila’s numerous technical advantages for the neurobiology of aggression. Acknowledgements: We are
very grateful to Martin Heisenberg for providing labspace, flies, equipment,
intellectual support and comments on the manuscript, to Dieter Dudacek
for immunohistochemistry, to Troy Zars for providing the P[GAL4] driver
and UAS toxin lines, to Maria Monastirioti for providing the M18 stocks,
to Wendy Neckameyer for most helpful discussions on the use of dopamine
pharmacology, to Jay Hirsh for advice on serotonin and dopamine mutants,
to Marcus Reif for helpful support on food-patch medium, to Reinhard
Wolf for supplying ‘Apfelmost’ for the food-patch medium, to David Pettigrew,
Randall Hayes, Gregg Phares, Gabi Putz, Robin Hiesinger, Sean McGuire
and Douglas Armstrong for discussion and comments on the manuscript
and to Hans Kaderaschabek and his team for designing and producing our
special equipment. Our special gratitude goes out to Edward Kravitz.
This study would probably never have been initiated if it wasn’t for
his curiosity and enthusiastic support. We owe much to his constant
feedback and his participation in our very constructive discussions. References:
Authors: Andrea Baier*, Britta Wittek* and Björn Brembs Full reference: Baier A.; Wittek B. and Brembs B. (2002): Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 205 (9): 1233–1240. (PDF) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||